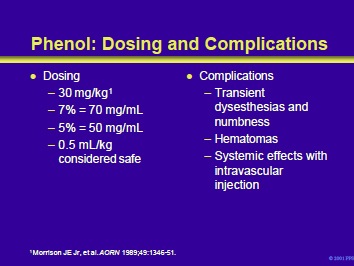
Morrison et al recommended a phenol dose of 30 mg/kg. A 5% and 7% solution of phenol contains 50 and 70 mg of phenol/mL, respectively. Therefore, when injecting a 30 kg child, the total dose should not exceed 900 mg. This can be translated into 18 mL of a 5% solution, or almost 13 mL of a 7% solution. On a volumetric basis, 0.5 mL/kg is considered to be within the limits of safety. Therefore, a 30 kg child should receive a volume of <15 mL (best supplied with a 7% solution). Injections can be repeated within days of each other.
Complications of phenol neurolysis include transient dysesthesias and numbness. The risk may be as high as 15% and is higher when mixed motor/sensory nerves are injected. Sensory complications are significantly less or nonexistent when pure motor nerves such as the obturator nerve (hip adductors) or musculocutaneous nerve (biceps) are treated. Intramuscular hematomas can form.
The local effects of the hematoma can negate the benefits of the injection. In addition, large doses of phenol delivered into the systemic circulation can lead to tremors, convulsions, and cardiorespiratory collapse.
If administered incorrectly or in subcutaneous muscles there may be erythema, blistering and potential skin slough.
Morrison JE Jr, Hertzberg DL, Gourley SM, et al. Motor point blocks in children. A technique to relieve spasticity using phenol injections. AORN 1989;49:1346-51.
Article Index
Page 9 of 11
Add comment