- Lectures / Webinars
- 8. Botulinum Toxin
8. Botulinum Toxin
Hot

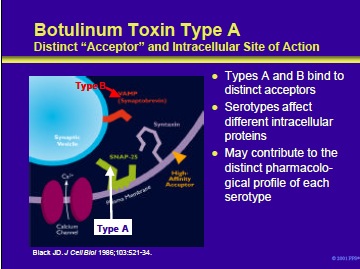
This slide shows an expanded view of a nerve terminal, depicting an extracellular acceptor and some of the intracellular proteins affected by the various botulinum neurotoxin serotypes. Botulinum toxin serotypes A and B bind to distinct acceptors on the external membrane of cholinergic neurons. These are the only two serotypes for which the acceptors have been well studied.
Once inside the nerve terminal, botulinum neurotoxins act by cleaving one or more of the intracellular proteins needed for neurotransmitter release. • Botulinum neurotoxin types A, C1 and E each cleave SNAP-25 at different sites •
Botulinum toxin types B, D, F, and G each cleave VAMP (synaptobrevin) at different sites • Type C1 also cleaves the protein syntaxin It is likely that these differences contribute to the distinct pharmacological profiles of the various serotypes (detailed in subsequent slides).
Black JD, Dolly JO. Interaction of 125-I labeled botulinum neurotoxins with nerve terminals. I. Ultrastructural autoradiographic localization and quantitation of distinct membrane acceptors for types A and B on motor nerves. J Cell Biol 1986;103:521-34.
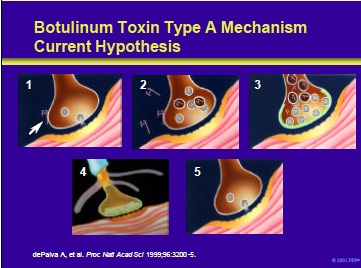
Botulinum toxin type A mechanism: current hypothesis. At the nerve terminal, botulinum toxin type A is thought to induce a temporary chemodenervation through the following steps:
- The toxin binds to acceptors (yet to be identified) on cholinergic terminals (arrow indicates a BTX-A molecule).
- The molecule is internalized into the nerve ending.
- Once inside the nerve ending, botulinum toxin interferes with the exocytosis of cholinergic vesicles. This leads to chemodenervation and reduced muscular contractions.
- Over time, terminal sprouting occurs.
- Finally, the original functional endplate is re-established and sprouts regress (as reported by Prof. Oliver Dolly’s laboratory). At this point symptoms may return in some patients.
Note: Data obtained from murine neuromuscular junction after a single BTX-A treatment revealed that the original endplate became reactivated as the sprouts regressed (dePaiva et al, 1999).
dePaiva A, Meunier FA, Molgo J, Aoki KR, Dolly JO. Functional repair of motor endplates after botulinum neurotoxin type A poisoning: biphasic switch of synaptic activity between nerve sprouts and their parent terminals. Proc Natl Acad Sci 1999;96:3200-5.

Uses of BTX therapy in the patient with cerebral palsy include the following:
- As an agent to decrease deformity in a growing child by modifying dynamic forces
- To modify the dynamic phase of deformity from ages 1 to 7 years
- To potentially improve muscle growth
- To protect released or transferred muscles during the immediate postoperative period
- To improve function during the acquisition phase of motor development
- To potentially improve motor control by decreasing focal spasticity
- To control postoperative pain

BTX therapy also has adjunctive uses in the patient with cerebral palsy. These include:
- To improve the process and results of physical therapy and/or occupational therapy. For example, the gastrocnemius can be injected to improve toe-toe gait.
- To facilitate the fitting and role of orthoses. For example, it can be used if an ankle-foot orthosis is unable to control plantar flexion.
- To alter dynamic forces during serial splinting/casting procedures. For example, it may be used in conjunction with casting or may obviate the need to serially cast ankles to gain a braceable foot.
- To modify hyperactive muscles for timed orthopedic surgical interventions.
- To facilitate the identification of realistic goals of antispasticity interventions

At this time, there are two commercially available botulinum toxins in the United States: BOTOX® (type A) and Myobloc® (type B). However, Dysport® (type A) is under consideration for licensing by the United States Food and Drug Administration. The neurotoxins differ in potency, formulation, and manufacturing. Each BOTOX® vial contains approximately 100 units, Myobloc® is dispensed in vials of 2,500, 5,000, and 10,000 units, and each Dysport® vial contains approximately 500 units.
Furthermore, 1 BOTOX® unit is the equivalent of 3 to 5 Dysport® units and is approximately the equivalent of 50 to 100 units of Myobloc®.

The BTX molecule is antigenic and has the potential to produce both neutralizing and nonneutralizing antibodies. However, the protein added to the vial to stabilize the preparation can act as an adjuvant; therefore, the total protein concentration is critical in determining the product’s potential immunogenicity. Furthermore, the total number of BTX units injected into the patient is another important factor determining the potential for antibody development.
The protein concentration in the current lots of BOTOX® is significantly less than the prior lot and, theoretically, should be less antigenic. It should be noted that antibody development has been primarily reported from studies on dystonia and not in patients with cerebral palsy and spasticity. In addition, caution should be exercised while interpreting this data since much of it is either retrospective or anecdotal.
It is the impression of the faculty that resistance due to antibody development is not a significant problem in treating children with cerebral palsy and spasticity. Instead, prior to attributing treatment failure to immunologic mechanisms, the treating physician should consider the possibilities that the wrong muscle was injected, the dose was incorrect, or the drug was inactivated by improper storage or handling.
When considering the possibility of resistance, the following precautions should be considered:
- Use the smallest possible dose
- Extend the interval between treatments as long as possible (³3 months)
- Avoid “booster” injections • Keep the Unit dose low (eg, £400 U)

BOTOX® must be kept frozen in the vial until approximately 1/2 hour prior to use. It is then reconstituted with normal saline (no preservative) to the desired concentration without shaking. Injection supplies should include appropriately sized syringes and needles and an EMG machine or electrical stimulator to localize the target muscle(s).
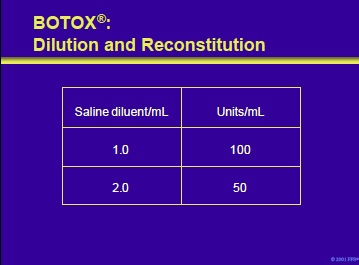
Prior to administration, BOTOX® must be reconstituted with sterile, preservative-free saline. The volume of diluent added determines the Unit dosage of BOTOX® per mL. In patients with cerebral palsy, concentrations of 100 U/mL are commonly employed. Dilution and reconstitution to produce 50 and 100 U/mL concentrations are illustrated on the slide.
The faculty recommends that the BOTOX® dose be referred to by Units rather than volume (eg, 100 U, not 1.0 mL).

The number of injections and dose require clinical judgment. The BOTOX® should be diluted to a concentration of 100 U/mL. By consensus, the faculty recommends 12-15 U/kg as a safe total body dose but agree that higher doses can be used with caution. The dose stated above is the total amount to be distributed in many muscles in the patient with cerebral palsy.
For injection of a single muscle, doses of 1-2 U/kg and 3-6 U/kg per site are considered safe for small and large muscles, respectively. The maximum dose per injection site should not exceed 50 U in a volume of 0.5 mL. The reinjection interval should be ³3 months to avoid resistance considerations.
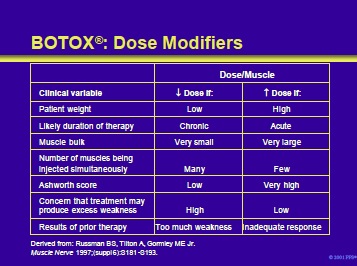
Clinical considerations that may influence the dose of BOTOX® administered to an individual patient are listed in the table on this slide.
Russman BS, Tilton A, Gormley ME Jr. Cerebral palsy: a rational approach to a treatment protocol, and the role of botulinum toxin in treatment. Muscle Nerve 1997;20 (suppl 6):S181-93.
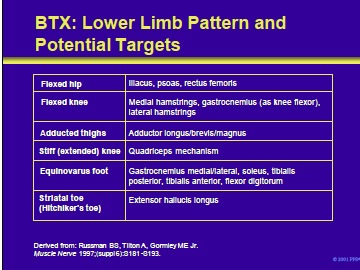
Patterns of spasticity of the lower limb and target muscles in patients with cerebral palsy that can be successfully treated with BTX include the following:
- Flexed hip
- Flexed knee
- Adducted thighs
- Stiff (extended) knee
- Equinovarus foot
- Striatal toe (hitchhiker’s toe)
Russman BS, Tilton A, Gormley ME Jr. Cerebral palsy: a rational approach to a treatment protocol, and the role of botulinum toxin in treatment. Muscle Nerve 1997;20 (suppl 6):S181-93.
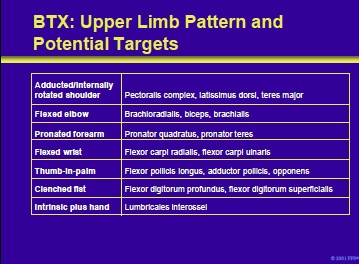
Patterns of spasticity of the upper limb and target muscles in patients with cerebral palsy that can be successfully treated with BTX include the following:
- Adducted/internally rotated shoulder
- Flexed elbow
- Pronated forearm
- Flexed wrist
- Thumb-in-palm
- Clenched fist
- Intrinsic plus hand

Botulinum toxin can diffuse through fascial planes to affect adjacent muscles that are not primary targets. For example, diffused toxin that affects the cholinergic innervation of the detrusor musculature can produce loss of bladder control.
Rare reported reactions include a flu-like syndrome, excessive weakness, rare cutaneous reactions, dry mouth, a brachial plexopathy, and polyradiculoneuritis. However, there have been no reports of adverse effects in patients with cerebral palsy and spasticity treated with BOTOX®.
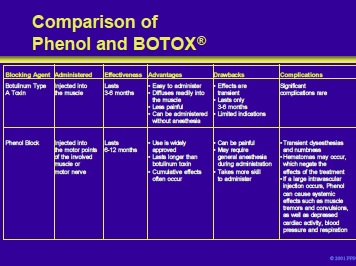
This slide compares neurolysis with phenol, and chemodenervation with BTX-A by effectiveness, advantages, drawbacks, and potential complications.
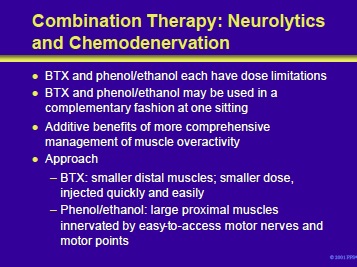
BTX and phenol or ethanol can be used together in an complementary approach to the patient with cerebral palsy and spasticity. This combined approach has the potential to avoid the dose limitations of each agent and offers the advantage of a more comprehensive management of muscle overactivity.
For example, BTX can be used to treat spasticity of small muscle groups while phenol or ethanol is used to control the manifestations of hyperactivity in larger muscle groups.
- See Next section: Neurosurgical Interventions for Spasticity in Children with Cerebral Palsy
Add comment