- Lectures / Webinars
- 6. Intrathecal Baclofen for Spasticity in Cerebral Palsy
6. Intrathecal Baclofen for Spasticity in Cerebral Palsy
Hot

Intrathecal baclofen (ITB) therapy employs an implantable pump to deliver measured amounts of baclofen into the cerebrospinal fluid in a timed fashion. This therapeutic approach can be used to treat patients with cerebral palsy and refractory spasticity.
The key to successful employment of ITB is the proper selection of candidates. Patients should be clinically stable, have severe multifocal and regional muscle overactivity and have failed an adequate trial of oral agents. Implantation is body -size dependent.
Consequently, patients are usually 4 years of age or older and weigh more than 30 pounds. However, severely affected patients not meeting these criteria may sometimes be treated.
The patient and the caregiver should have realistic goals for the outcomes of therapy. In addition, they should be motivated and committed to ensure the success of ITB therapy.
Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.

Intrathecal baclofen should not be considered when any infection is present, if the patient has a history of allergy or hypersensitivity to the drug, or if there is any chance of pregnancy or breast-feeding.
Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.
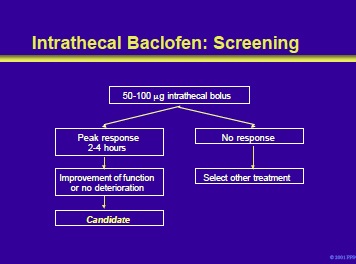
Patients who meet the criteria for ITB should be screened by the algorithm shown on the slide. Screening consists of the administration of a bolus of intrathecal baclofen by lumbar puncture or spinal catheter. Most patients will respond to the initial dose of 50 mg. A response consists of a significant reduction in spasticity, usually within 30 minutes to 1 hour, and peaking at 2 to 4 hours. In those who do not respond to the initial bolus, a trial with 75 mg or 100 mg is the next step.
During this process, patients should be monitored carefully for any signs of a drug overdose or other adverse effects. Failure to respond to a dose £100 mg indicates that the patient is not a candidate for intrathecal baclofen.
References
- Albright AL. Intrathecal baclofen in cerebral palsy movement disorders. J Child Neurol 1996;11(Suppl 1):S29-S35.
- Becker R, Alberti O, Bauer BL, et al. Continuous intrathecal baclofen infusion in severe spasticity after traumatic or hypoxic brain injury. J Neurol 1997;240:160-6.
- Coffey RJ, Cahill D, Steers W, et al, for the United States Intrathecal Baclofen Study Group. Intrathecal baclofen for intractable spasticity of spinal origin: results of a long-term multicenter study. J Neurosurg 1993;78:226-32.
- Ordia JI, Fisher E, Asamski E, et al. Chronic intrathecal delivery of baclofen by a programmable pump forfor the treatment of severe spasticity. J Neurosurg 1996;85:452-7.
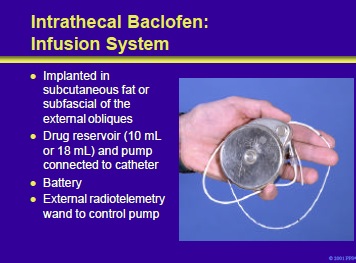
The baclofen infusion system consists of an implantable pump with either a 10- or 18-mL reservoir, a spinal catheter, a battery and an external programmer to adjust drug delivery.
Reference:
Albright AL. Intrathecal baclofen in cerebral palsy movement disorders. J Child Neurol 1996;11(suppl 1):S29-S35.
Albright AL, Barron WB, Fasick MP, et al. Continuous intrathecal baclofen infusion for spasticity of cerebral origin. JAMA 1993;270:2475-9.
Penn RD, Savoy SM, Corcos D, et al. Intrathecal baclofen for severe spinal spasticity. N Engl J Med 1989;320:1517-21.
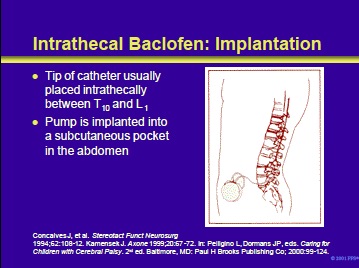
The surgery may be performed under local or general anesthesia. Operative times vary from 1 to 1-1/2 hours. The pump is inserted into a pocket that is surgically created in the subcutaneous skin of the abdomen.
A one-piece radiopaque catheter is advanced into the lumbar subarachnoid space and advanced until the tip is between T10 and L1. In selected patients treated in referral centers, catheters have been placed in the cervical region, or even as high as the cisterna magnum. The catheter is tunneled around the abdomen connecting to the implanted pump.
References
- Concalves J, Barcia-March G, Sanchez-Ledesma MJ, et al. Management of intractable spasticity of supraspinal origin by chronic cervical intrathecal infusion of baclofen. Stereotact Funct Neurosurg 1994;62:108-12.
- Kamensek J. Continuous intrathecal baclofen infusions: an introduction and review. Axone 1999;20:67-72.
- Ried S, Pellegrino L, Albinson-Scull S, et al. The Management of Spasticity. In: Pelligino L, Dormans JP, eds. Caring for Children with Cerebral Palsy. 2nd ed. Baltimore, Md: Paul H Brooks Publishing Co; 2000:99-124.

The dose is titrated to provide the patient with enough spasticity to be useful in balance and positioning without being disabling. Prior to discharge, patients and their caregiver(s) are instructed on the technology, refill schedule, potential complications and adverse effects. Patient or caregiver should be supplied with oral baclofen or diazepam suppositories to manage pump dysfunction or failure.
Following implantation, aggressively attempt to increase range of motion (ROM), ambulation and gross motor skills. Night splinting and knee immobilizers can be employed to help increase ROM. Therapies can be slowly decreased to a more routine basis (ie, pre-pump implantation) after three months. Routine care is provided by the patient’s primary care physician or the referring physician.
Refills, assessments and possible dose adjustments are performed at the ITB therapy center at 4 to 12 week intervals.
Dose adjustments are based on the degree of muscle tone, severity, and frequency of spasms, presence of adverse drug effects, patient-related conditions, functional assessment and system functions. The pump should be replaced after 5 to 7 years.
Albright AL. Intrathecal baclofen in cerebral palsy movement disorders. J Child Neurol 1996;11(suppl 1):S29-S35.
Complications of ITB can be local or systemic. Local complications include the accumulation of serous fluid or blood at the surgical site, erosion of the pump through the skin or infection. Systemic complications include drug withdrawal (eg, increased spasticity, hallucinations, seizures) or drug toxicity (eg, drowsiness, dizziness, somnolence, respiratory depression, coma).
Other potential complications include meningitis, urinary retention/constipation and possible progression of scoliosis. Complications specifically related to the catheter can also occur. These include migration of the catheter, interruption of the flow of baclofen from the reservoir due to breakage, puncture/rupture, dislodgment or disconnection.
In addition, leaks of cerebrospinal fluid may occur.
Kamensek J. Continuous intrathecal baclofen infusions: an introduction and overview. Axone 1999;20:67-72.
- Next section: Local Anesthetics, Neurolytics, and Chemodenervation for the Patient with Cerebral Palsy and Spasticity
Add comment