- Details
- ICNA
- News
- Hits: 952
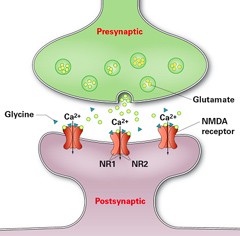
In a study have been published in the journal Neurology researchers from Charité - Universitätsmedizin Berlin and the German Center for Neurodegenerative Diseases (DZNE) using a new treatment regimen have recorded significant progress in patients with Anti-NMDA receptor encephalitis. In addition to standard treatment, patients received bortezomib, a drug known as proteasome inhibitor that has proven successful in treating patients with plasmacytoma a haematological malignancy. Proteasomes play an important role in the degradation of proteins that regulate the cell cycle, thereby regulating cell growth. Given their high rates of protein synthesis, antibody-producing plasma cells display particularly high levels of metabolic activity. This renders them as sensitive to the effects of the drug as cancer cells, and results in their death.
Five severely affected patients with anti-NMDAR encephalitis with delayed treatment response or resistance to standard immunosuppressive and B-cell-depleting drugs (corticosteroids, IV immunoglobulins, plasma exchange, immunoadsorption, rituximab, cyclophosphamide) who required medical treatment and artificial ventilation on intensive care units were treated with 1–6 cycles of 1.3 mg/m2 bortezomib
Bortezomib treatment showed clinical improvement or disease remission, which was accompanied by a partial NMDAR antibody titer decline in 4 of 5 patients. With respect to disease severity, addition of bortezomib to the multimodal immunosuppressive treatment regimen was associated with an acceptable safety profile.
"'Bortezomib is capable of treating the causes of the disease by eliminating plasma cells. This makes it a valuable new treatment option in cases of anti-NMDA receptor encephalitis that have so far proven resistant to treatment," explains Franziska Scheibe, the study's first author. The results need to be confirmed by randomized trials as is the case with all the other treatment currently used in anti NMDA receptor encephalitis patients.
Read More
- Details
- ICNA
- News
- Hits: 859
A systematic review of the evidence confirms that infection with mosquito-borne Zika virus is a cause of Guillain-Barre Syndrome (GBS), in addition to microcephaly and other congenital brain abnormalities, according to a study published in PLOS Medicine by Nicola Low of the University of Bern, Switzerland, and colleagues in the World Health Organization (WHO) Zika Causality Working Group.
In March 2016, WHO stated that there was a strong scientific consensus that Zika virus is a cause of GBS, microcephaly, and other neurological disorders. However, decisions about causality must be assessed systematically to guide public health actions. In the new work, the WHO group defined specific questions about the relationship between Zika virus and clinical outcomes, setting a framework of ten dimensions to define causality. They then reviewed existing literature for studies that addressed the outcomes - either GBS or congenital brain abnormalities - and convened a panel of experts to assess the review findings.
Based on 72 studies published up to May 30, 2016, that included data on Zika and congenital brain abnormalities, the team concluded that at least eight of the ten criteria for causality were met. Based on 36 studies published in the same time frame with data on Zika and GBS, the researchers concluded that at least seven of the ten criteria for causality were met. In addition, papers published after the initial literature review - between May 30 and July 29, 2016 - strengthened the initial findings that Zika virus is causative of brain abnormalities and GBS.
Rapid and systematic reviews with frequent updating and open disseminating are now needed, both for appraisal of the evidence about Zika virus infection and for the next public health threats that will emerge
However, there are remaining questions about the link that will need to be addressed with continuing studies. The review was funded by the World Health Organization (http://www.who.int/en/, contract numbers 2016/611294-0 and 2016/630126-0 awarded to NL) and the Swiss National Science Foundation (www.snf.ch, SNSF special action fund and project grant 320030_170069 awarded to NL). The WHO Zika Causality Working Group was involved in interpretation of the data and the decision to publish.
Article: Zika Virus Infection as a Cause of Congenital Brain Abnormalities and Guillain-Barré Syndrome: Systematic Review, Krauer F, Riesen M, Reveiz L, Oladapo OT, Martínez-Vega R, Porgo TV, et al., PLOS Medicine, doi: 10.1371/journal.pmed.1002203, published 3 January 2016...
Read More
- Details
- ICNA
- News
- Hits: 920
SPINRAZA (nusinersen) is an antisense oligonucleotide (ASO) that is designed to treat SMA caused by mutations in the chromosome 5q that leads to SMN protein deficiency. SPINRAZA (nusinersen) alters the splicing of SMN2 pre-mRNA in order to increase production of full-length SMN protein. ASOs are short synthetic strings of nucleotides designed to selectively bind to target RNA and regulate gene expression. Through use of this technology, SPINRAZA (nusinersen) has the potential to increase the amount of full-length SMN protein in patients with SMA.
SPINRAZA (nusinersen) is administered via intrathecal injection, which delivers therapies directly to the cerebrospinal fluid (CSF) around the spinal cord, where motor neurons degenerate in patients with SMA due to insufficient levels of SMN protein.
The most common adverse reactions reported for SPINRAZA (nusinersen) were upper respiratory infection, lower respiratory infection and constipation. Serious adverse reactions of atelectasis were more frequent in SPINRAZA (nusinersen)-treated patients. Coagulation abnormalities and thrombocytopenia, including acute severe thrombocytopenia, have been observed after administration of some antisense oligonucleotides.
Renal toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides.
SMA360°
Surrounding you with support
Read More
- Details
- ICNA
- News
- Hits: 887
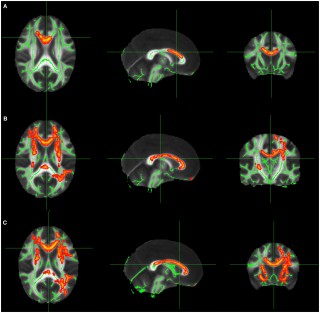
In research presented at the 2016 American Epilepsy Society annual meeting in Houston University of Munich Hospital's epilepsy center and its department of neuroradiology reported on their findings from using DTI to locate epileptogenic abnormalities. The technique is already being regularly used to guide implantation of intracranial electrodes at the University of Munich. The DTI technique involves quantification of regional U-fibers to identify microstructural abnormalities in patients with cryptogenic focal epilepsy (cFE).
The most frequent pathology in cases with cryptogenic focal epilepsy is focal cortical dysplasia, which is typically located at the gray-white-matter border, typically in the bottom of a sulcus, and this is where U-fibers are located. The density of U-fibers we analyzed is therefore a specific neuroimaging biomarker for the gray-white-matter border and seems to be very sensitive to alterations caused by subtle forms of focal cortical dysplasia.
They studied 55 cFE patients and 60 healthy controls. Whole-brain tractography by DTI was performed, and tracts representing subcortical U-fibers were selected based on length, curvature, and shape. Tract density images were then created, specifying the number of tracts passing through any voxel. Clusters of significant reductions in U-fiber density were compared with clinical data from presurgical evaluation, including ictal and interictal electroencephalography (EEG), seizure semiology, fluorodeoxyglucose-positron emission tomography (FDG-PET), and ictal single-photon emission computed tomography imaging (SPECT).
Finaly analysis was done on 43 patients where detailed clinical data was available, clusters of significant U-fiber reductions were found in 91 percent of the patients, and 78 percent were consistent with the clinical localization of the epileptogenic zone. Twelve patients underwent intracranial EEG recording with depth electrodes, confirming seizure onset in 74 percent of the detected U-fiber reductions. Eight patients had resective surgery, and the most frequent histopathologic finding was mild malformation of cortical development, with blurred gray-white matter border and ectopic neurons in the white matter.
According to Christian Vollmar, MD, PhD at the University of Munich, U-fiber quantification is definitely good enough to base further investigations on, particularly to plan the implantation of invasive electrodes, and to give it the same weight that other established methods like ictal SPECT or FDG-PET. No single diagnostic modality alone is ever good enough to base surgical decisions on in case of cryptogenic focal epilepsy." It is important to note that this report requires validation in larger, prospective studies, with outcome data to substantiate whether the technique ultimately results in fewer seizures following surgery. In addition it also relies on accurate DTI mapping with the parameters set in just the right way.
- AES Abstract 3.196: Vollmar C, Goc J, Hartl E, Noachtar S. U-fiber track density imaging identifies specific alterations in the epileptogenic zone in individual patients with cryptogenic focal epilepsy.
- Just pretty pictures? What diffusion tractography can add in clinical neuroscience - Heidi Johansen-Berg and Timothy E.J. Behrens
Read More

- Details
- ICNA
- News
- Hits: 1041
Ich bin schon ein toller Typ (I am already a great guy)
Dieter Janz himself joked when he addressed the guests at his 90th birthday celebrations : "After all that I have heard about myself today, I must say: I am already a great guy" - impressed by himself after all the praise , And especially thanked his wife Gabriele for 62 common years of marriage.
Akademische Feier zum 90. Geburtstag des Epileptologen Professor Dieter Janz, Emeritus der Freien Universität
Read More