Conference Title: 8th National Conference of the Association of Child Neurology " Bridging Advances and Basics in Child Neurology "
Location: New Delhi, India Date: Feb. 3-5, 2017 Hosting Society: Association of Child Neurology
Local Hosts: Dr. Rekha Mittal, Dr. Suvasini Sharma, Dr. Rakesh Jain
Type of meeting: National Conference
Participants: The 8th National conference of the Association of Child Neurology hosted approximately 400 attendees including 275 delegates (child neurologists, adult neurologists, pediatricians and trainees) plus 90 faculty. The delegates originated from all regions of India and included a few delegates from Bangladesh and Nepal. The faculty included 12 international and 78 national faculty.
Panel of speakers and speaker affiliations:
1. Linda De Meirleir, Vrie Universiteit Brussels, Brussels, Belgium
2. Biju Hameed, Bristol Royal Hospital for Children, University of Bristol, Bristol, UK
3. Michael Johnston, Kennedy-Krieger Institute and Children's Hospital and Johns Hopkins Hospital, Baltimore, USA
4. Pratibha Singhi, Advanced Pediatrics Center, Post Graduate Institute of Medical Education and Research, Chandigarh, India
5. Ingrid Tein, Hospital for Sick Children, University of Toronto, Toronto, Canada
6. Jo Wilmshurst, Red Cross Children's Hospital, University of Capetown, Capetown, South Africa
Topics covered (and speaker)
1. Linda De Meirleir: Pyridoxine dependency and related disorders.
2. Biju Hameed: Rational treatment of epilepsy and newer anti-epileptic drugs (panel discussion)
3. Michael Johnston: Disease modifying treatments for neurogenetic developmental disorders – what does the future hold ?
4. Pratibha Singhi: Dyskinetic cerebral palsy – recent advances in diagnosis and treatment.
5. Ingrid Tein: Metabolic myopathies; Recent advances in the treatment of mitochondrial disorders
6. Jo Wilmshurst: Epilepsy in infants: Treatment guidelines.
The conference was composed of parallel sessions including the following themes (1) New frontiers in pediatric neurology (2) Hot topics in epilepsy (3) Status epilepticus (4) Bedside clinical neurology (5) Immune mediated and inflammatory disorders (6) Video sessions of autism, reflex seizures, psychogenic seizures and epilepsy mimics in infants (7) Neuromuscular disorders (8) Neurometabolic disorders (9) Recent advances in pediatric neurology (10) Epilepsy (11) Neuro-infections (12) Stroke and headache.
The common sessions included (1) Panel discussion on the rational treatment of epilepsy and newer anti-epileptic drugs (2) Mechanisms of epilepsy (3) Neurodevelopmental disorders (4) Genetic testing in pediatric neurologic conditions (5) Debate on Neonatal screening (6) Parasomnias (7) Movement disorders (8) Controversies in neurology. There were also poster presentations with a poster competition adjudicated by national faculty and ICNA members.
Outcomes/Future Plans
1. The conference stimulated lively interactive academic discussions.
2. Excellent progress was made during the site visit and in the planning of the scientific program for the upcoming ICNC2018 (Nov. 14-18th, 2018) in Mumbai, India with the local organizing committee and executive board of the AOCN.
Support:
According to the ICNA policy, the ICNA members supported their own international flights as well as their own accomodations in Mumbai during the ICNC2018 site visit. The ICNA members gratefully acknowledge the support of the AOCN for the internal costs of accomodations and local travel related to the AOCN conference in New Delhi.
ICNA members received no honoraria. The ICNA members also acknowledge their respective Universities/teaching hospitals for allowing them to use their conference leave time away from service commitments to contribute to the AOCN conference.
Main conference program http://www.childneurocon2017.com/scientific-program/
Read More
- Details
- Ingrid Tein
- News
- Hits: 1000
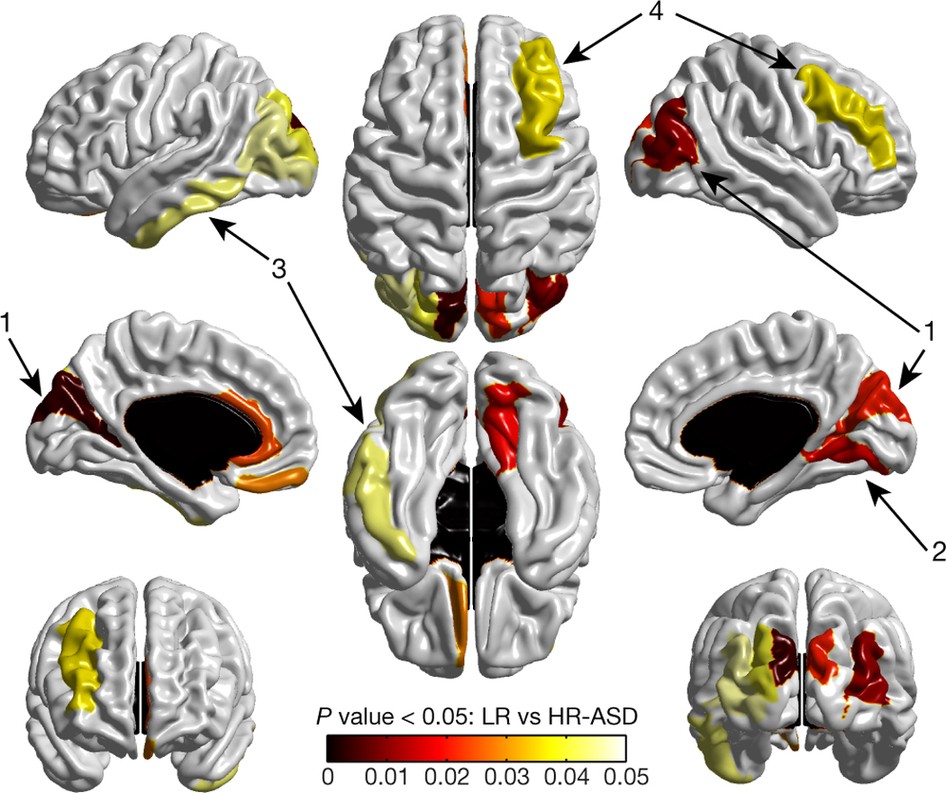
Using magnetic resonance imaging (MRI) in infants with older siblings with autism, researchers from around the country were able to correctly predict 80 percent of those infants who would later meet criteria for autism at two years of age.
The study, published today in Nature, is the first to show it is possible to identify which infants – among those with older siblings with autism – will be diagnosed with autism at 24 months of age. This first-of-its-kind study used MRIs to image the brains of infants, and then researchers used brain measurements and a computer algorithm to accurately predict autism before symptoms set in.
"Our study shows that early brain development biomarkers could be very useful in identifying babies at the highest risk for autism before behavioral symptoms emerge," said senior author Joseph Piven, MD, the Thomas E. Castelloe Distinguished Professor of Psychiatry at the University of North Carolina-Chapel Hill. "Typically, the earliest an autism diagnosis can be made is between ages two and three. But for babies with older autistic siblings, our imaging approach may help predict during the first year of life which babies are most likely to receive an autism diagnosis at 24 months."
This research project included hundreds of children from across the country and was led by researchers at the Carolina Institute for Developmental Disabilities (CIDD) at the University of North Carolina, where Piven is director. The project's other clinical sites included the University of Washington, Washington University in St. Louis, and The Children's Hospital of Philadelphia. Other key collaborators are McGill University, the University of Alberta, the University of Minnesota, the College of Charleston, and New York University.
"This study could not have been completed without a major commitment from these families, many of whom flew in to be part of this," said first author Heather Hazlett, PhD, assistant professor of psychiatry at the UNC School of Medicine and a CIDD researcher. "We are still enrolling families for this study, and we hope to begin work on a similar project to replicate our findings."
People with Autism Spectrum Disorder (or ASD) have characteristic social deficits and demonstrate a range of ritualistic, repetitive and stereotyped behaviors. It is estimated that one out of 68 children develop autism in the United States. For infants with older siblings with autism, the risk may be as high as 20 out of every 100 births. There are about 3 million people with autism in the United States and tens of millions around the world.
Despite much research, it has been impossible to identify those at ultra-high risk for autism prior to 24 months of age, which is the earliest time when the hallmark behavioral characteristics of ASD can be observed and a diagnosis made in most children.
For this Nature study, Piven, Hazlett, and researchers from around the country conducted MRI scans of infants at six, 12, and 24 months of age. They found that the babies who developed autism experienced a hyper-expansion of brain surface area from six to 12 months, as compared to babies who had an older sibling with autism but did not themselves show evidence of the condition at 24 months of age. Increased growth rate of surface area in the first year of life was linked to increased growth rate of overall brain volume in the second year of life. Brain overgrowth was tied to the emergence of autistic social deficits in the second year.
Previous behavioral studies of infants who later developed autism – who had older siblings with autism –revealed that social behaviors typical of autism emerge during the second year of life.
The researchers then took these data – MRIs of brain volume, surface area, cortical thickness at 6 and 12 months of age, and sex of the infants – and used a computer program to identify a way to classify babies most likely to meet criteria for autism at 24 months of age. The computer program developed the best algorithm to accomplish this, and the researchers applied the algorithm to a separate set of study participants.
The researchers found that brain differences at 6 and 12 months of age in infants with older siblings with autism correctly predicted eight out of ten infants who would later meet criteria for autism at 24 months of age in comparison to those infants with older ASD siblings who did not meet criteria for autism at 24 months.
"This means we potentially can identify infants who will later develop autism, before the symptoms of autism begin to consolidate into a diagnosis," Piven said.
If parents have a child with autism and then have a second child, such a test might be clinically useful in identifying infants at highest risk for developing this condition. The idea would be to then intervene 'pre-symptomatically' before the emergence of the defining symptoms of autism.
Research could then begin to examine the effect of interventions on children during a period before the syndrome is present and when the brain is most malleable. Such interventions may have a greater chance of improving outcomes than treatments started after diagnosis.
"Putting this into the larger context of neuroscience research and treatment, there is currently a big push within the field of neurodegenerative diseases to be able to detect the biomarkers of these conditions before patients are diagnosed, at a time when preventive efforts are possible," Piven said. "In Parkinson's for instance, we know that once a person is diagnosed, they've already lost a substantial portion of the dopamine receptors in their brain, making treatment less effective."
Piven said the idea with autism is similar; once autism is diagnosed at age 2-3 years, the brain has already begun to change substantially.
"We haven't had a way to detect the biomarkers of autism before the condition sets in and symptoms develop," he said. "Now we have very promising leads that suggest this may in fact be possible."
For this research, NIH funding was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Institute of Mental Health (NIMH), and the National Institute of Biomedical Imaging and Bioengineering. Autism Speaks and the Simons Foundation contributed additional support.
Source: UNC Health Care Press Release
Citation: Hazlett HC, Gu H, Munsell BC, Kim SH, Styner M, Wolff JJ et al. (2017) Early brain development in infants at high risk for autism spectrum disorder. Nature 542 (7641):348-351. DOI: 10.1038/nature21369 PMID: 28202961.
Cover image: A map of significant group differences in surface area from 6 to 12 months. Exploratory analyses were conducted with a surface map containing 78 regions of interest
Read More
- Details
- ICNA
- News
- Hits: 1134

A unique meeting on "Brain Plasticity in Epilepsy" will be held in Leuven, Belgium, May 13 – 16, 2017. Our goal is to provide a highly interactive forum for clinical practitioners and researchers with a focus on brain reorganization in epilepsy and how brain plasticity affects motor, cognitive and imaging aspects. We are very honored to be hosting an expert faculty of distinguished international speakers on the following topics: brain networks in epilepsy epileptogenesis and functional plasticity brain reorganization in epilepsy: motor, cognitive and imaging aspects disconnection in epilepsy neuromodulation prediction of seizures and outcomes We encourage you to submit an abstract and share your research with other experts in the field of epilepsy.
Epilepsy is a network disorder of the brain characterized by an enduring predisposition to generate epileptic seizures and the neurobiologic, cognitive and psychosocial consequences of this condition. Time is ripe for a reunion of neurologists, neurosurgeons, imaging specialists, neuropediatrricians and neuroscientists to address the role of brain plasticity in epilepsy. We will review plastic changes in brain networks in epilepsy, animal models of epileptogenesis, and how we could influence these processes. We will focus on brain reorganization in epilepsy and how brain plasticity affects motor, cognitive and imaging aspects. We will address state-of-the-art disconnection procedures in epilepsy surgery, disconnection syndromes and how plastic changes after disconnections could affect outcome. Finally, we will focus on the dynamics of the epileptic brain and how we could use different modulation techniques to treat refractory epilepsy. This international symposium will feature advances in the conceptualization of these strategies and a better understanding of brain plasticity that should be integrated into clinical decision making.
The proceedings of the meeting will be published as review articles in Acta Neurologica Belgica.
Read More
- Details
- ICNA
- News
- Hits: 1118

This guideline covers diagnosing, assessing and managing cerebral palsy in children and young people from birth up to their 25th birthday. It aims to make sure they get the care and treatment they need for the developmental and clinical comorbidities associated with cerebral palsy, so that they can be as active and independent as possible
Recommendations This guideline includes recommendations on:
- causes and recognition of cerebral palsy
- multidisciplinary care and information and support
- managing feeding and drooling problems
- support with speech, language and communication
- assessing and managing pain, discomfort, distress and sleep disturbances
- information on other comorbidities, including mental health problems
- transition to adults' services
Picture courtesy: Inspirational Bailey Matthews was roared over the finishing line by crowds close to tears as he cast aside his specially-adapted walking frame to finish the last 20 metres of the race unaided at the Castle Howard Triathlon in North Yorkshire 2015
Read More
- Details
- ICNA
- News
- Hits: 1085
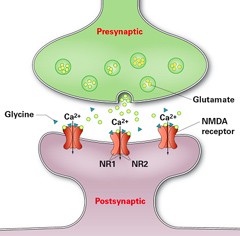
In a study have been published in the journal Neurology researchers from Charité - Universitätsmedizin Berlin and the German Center for Neurodegenerative Diseases (DZNE) using a new treatment regimen have recorded significant progress in patients with Anti-NMDA receptor encephalitis. In addition to standard treatment, patients received bortezomib, a drug known as proteasome inhibitor that has proven successful in treating patients with plasmacytoma a haematological malignancy. Proteasomes play an important role in the degradation of proteins that regulate the cell cycle, thereby regulating cell growth. Given their high rates of protein synthesis, antibody-producing plasma cells display particularly high levels of metabolic activity. This renders them as sensitive to the effects of the drug as cancer cells, and results in their death.
Five severely affected patients with anti-NMDAR encephalitis with delayed treatment response or resistance to standard immunosuppressive and B-cell-depleting drugs (corticosteroids, IV immunoglobulins, plasma exchange, immunoadsorption, rituximab, cyclophosphamide) who required medical treatment and artificial ventilation on intensive care units were treated with 1–6 cycles of 1.3 mg/m2 bortezomib
Bortezomib treatment showed clinical improvement or disease remission, which was accompanied by a partial NMDAR antibody titer decline in 4 of 5 patients. With respect to disease severity, addition of bortezomib to the multimodal immunosuppressive treatment regimen was associated with an acceptable safety profile.
"'Bortezomib is capable of treating the causes of the disease by eliminating plasma cells. This makes it a valuable new treatment option in cases of anti-NMDA receptor encephalitis that have so far proven resistant to treatment," explains Franziska Scheibe, the study's first author. The results need to be confirmed by randomized trials as is the case with all the other treatment currently used in anti NMDA receptor encephalitis patients.
Read More
- Details
- ICNA
- News
- Hits: 939