 Scientists at the University of Southern California (USC), Queen's University (Ontario) and Duke University publishing in Frontiers in Neurology describe a new tool that can screen children for fetal alcohol spectrum disorder (FASD) quickly and affordably, making it accessible to more children in remote locations worldwide.
Scientists at the University of Southern California (USC), Queen's University (Ontario) and Duke University publishing in Frontiers in Neurology describe a new tool that can screen children for fetal alcohol spectrum disorder (FASD) quickly and affordably, making it accessible to more children in remote locations worldwide.
The tool uses a camera and computer vision to record patterns in children's eye movements as they watch multiple one-minute videos, or look towards/away from a target, and then identifies patterns that contrast to recorded eye movements by other children who watched the same videos or targets. The eye movements outside the norm were flagged by the researchers as children who might be at-risk for having FASD and need more formal diagnoses by healthcare practitioners.
The study "Detection of Children/Youth With Fetal Alcohol Spectrum Disorder Through Eye Movement, Psychometric, and Neuroimaging Data," by Chen Zhang, Angelina Paolozza, Po-He Tseng, James N. Reynolds, Douglas P. Munoz and Laurent Itti, was published in Frontiers in Neurology.
According to the paper's corresponding author, Laurent Itti, a professor of computer science, psychology and neuroscience at USC, FASD is still quite difficult to diagnose -- a professional diagnosis can take a long time with the current work up taking as much as an entire day. "There is not a simple blood test to diagnose FASD. It is one of those spectrum disorders where there is a broad range of the disorder. It is medically very challenging and it is co-morbid with other conditions. The current gold standard is subjective, as it involves a battery of tests and clinical evaluation. It is also costly."
Itti said he and his colleagues conducted this research as they felt that a screening tool might be able to reach more children who might be at risk. It is estimated that millions of children will be diagnosed with fetal alcohol spectrum disorder (FASD). This condition, when not diagnosed early in a child's life, can give rise to secondary cognitive and behavioral disabilities.
"The new screening procedure only involves a camera and a computer screen, and can be applied to very young children. It takes only 10 to 20 minutes and the cost should be affordable in most cases," said Chen Zhang, a doctoral candidate from the Neuroscience Graduate Program at USC and the paper's first author. "The machine learning pipeline behind this gives out objective and consistent estimations in minutes."
While this computer vision tool is not intended to replace full diagnosis by professionals, it is intended to provide important feedback so that parents can ensure that their children are seen by professionals and receive early cognitive learning and potentially behavioral interventions.
The impact of such screening tools could be significant. Study co-author and FASD expert, James N. Reynolds, who is the Interim Chief Scientific Officer at the Kids Brain Health Network, said, "The economic impact of FASD is spread across multiple systems, including health care, education, criminal justice, as well as lost productivity costs for both individuals with FASD and their caregivers. Estimates suggest that the mean annual cost of FASD in Canada and the US ranges from $22,000-$24,000 per individual, and therefore many billions of dollars collectively on society. There is simply no escaping the fact that FASD is a major public health problem associated with tremendous economic and social costs."
USC's Itti has many ideas of how this screening tool could be implemented whether via a mobile unit, on an app or in a pharmacy as one of the free screening tools used while waiting for a prescription. He says, 'This could be the blood pressure monitoring system for your brain."
Funding for this work was provided by the Kids Brain Health Network [formerly NeuroDevNet; Reynolds et al.], the National Science Foundation (CCF-1317433 and CNS-1545089), and the JPB Foundation (grant number 378).
Article reference
Chen Zhang, Angelina Paolozza, Po-He Tseng, James N. Reynolds, Douglas P. Munoz, Laurent Itti. Detection of Children/Youth With Fetal Alcohol Spectrum Disorder Through Eye Movement, Psychometric, and Neuroimaging Data. Frontiers in Neurology, 2019; 10 DOI: 10.3389/fneur.2019.00080
Read More
- Details
- ICNA
- News
- Hits: 1066
Researchers from Trinity College Dublin publishing in BRAIN describes for the first time a model of mitochondrial epilepsy. Mitochondrial disease is one of the most common forms of genetic diseases, affecting one in 9,000 births in Ireland with debilitating consequences. A quarter of patients with mitochondrial disease have epilepsy which is often severe and resistant towards conventional antiepileptic drugs.
Currently no animal models are available to provide a mechanistic understanding of the condition. The current study has thrown light at the important role that astrocytes play in driving seizure generation in mitochondrial epilepsy. They recreated a novel brain slice model by the application of an astrocytic-specific aconitase inhibitor, fluorocitrate, concomitant with mitochondrial respiratory inhibitors, rotenone and potassium cyanide. The model was robust and exhibited both face and predictive validity.
The model was then used to assess the role that astrocytes play in seizure generation and demonstrated the involvement of the GABA-glutamate-glutamine cycle, which regulates how chemical transmitters are released from neurons and then taken up by the supporting cells; the astrocytes. Notably, glutamine appears to be an important intermediary molecule between the neuronal and astrocytic compartment in the regulation of GABAergic inhibitory tone.
They also found that a deficiency in glutamine synthetase is an important part of the pathogenic process for seizure generation in both the brain slice model and the human neuropathological study. Future work will develop the model so that it can be used to stratify novel anti-seizure drugs in a tailored manner for patients diagnosed with mitochondrial disorders and who phenotypically exhibit epilepsy."
Story Source: Trinity College Dublin
Journal Reference:
Felix Chan, Nichola Z Lax, Caroline Marie Voss, Blanca Irene Aldana, Shuna Whyte, Alistair Jenkins, Claire Nicholson, Sophie Nichols, Elizabeth Tilley, Zoe Powell, Helle S Waagepetersen, Ceri H Davies, Doug M Turnbull, Mark O Cunningham. The role of astrocytes in seizure generation: insights from a novel in vitro seizure model based on mitochondrial dysfunction. Brain, 2019; 142 (2): 391 DOI: 10.1093/brain/awy320
Read More
- Details
- ICNA
- News
- Hits: 1434
 Swiss drugmaker Novartis has received US approval for its spinal muscular atrophy gene therapy Zolgensma® (onasemnogene abeparvovec-xioiT) for the treatment of pediatric patients less than 2years of age with spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene). The one time treatment drug is priced at a record $2.125m. Novartis executives have defended the price, saying a one-time treatment is more valuable than expensive long-term treatments that cost several hundred thousand dollars a year.
Swiss drugmaker Novartis has received US approval for its spinal muscular atrophy gene therapy Zolgensma® (onasemnogene abeparvovec-xioiT) for the treatment of pediatric patients less than 2years of age with spinal muscular atrophy (SMA) with bi-allelic mutations in the survival motor neuron 1 (SMN1) gene). The one time treatment drug is priced at a record $2.125m. Novartis executives have defended the price, saying a one-time treatment is more valuable than expensive long-term treatments that cost several hundred thousand dollars a year.
Zolgensma® is designed to address the genetic root cause of SMA by providing a functional copy of the human SMN gene to halt disease progression through sustained SMN protein expression with a single, one-time intravenous (IV) infusion. Zolgensma represents the first approved therapeutic in a proprietary platform to treat rare, monogenic diseases using gene therapy.
|
"At this stage, the medical community is awaiting the results of ongoing trials in other forms of SMA such as type II and type III, as well as various means of delivery such as intrathecal Zolgensma. Beyond babies, it is not possible to deliver AAV intravenously, since antibodies against this virus may already be present by adolescence or early adulthood. Further some patients may need repeated dosages. It is important to remain cognizant that the safety profile for such interventions often needs time to become fully evident. As such the full characterisation of the respective safety profiles of new agents in the management of SMA I, II and III will require the careful and meticulous evaluation of the efficacy and adverse events through the ongoing Randomised Control Trials over time." |
The therapy uses a virus to provide a normal copy of the SMN1 gene to babies born with a defective gene and is delivered by infusion. Novartis said it has so far treated more than 150 patients with Zolgensma. Zolgensma according to Novartis is a near-cure for SMA if delivered soon after birth although current available data extends only to four years post therapy. European and Japanese approvals of the drug is expected laster this year.
The competitor drug Biogen’s Spinraza, was first approved treatment for SMA in 2016, but requires infusion into the spinal canal every four months and is priced at around $750,000 for the initial year and $375,000 annually thereafter.
SMA is a severe neuromuscular disease characterized by the loss of motor neurons leading to progressive muscle weakness and paralysis. SMA is caused by a genetic defect in the SMN1 gene that codes SMN, a protein necessary for survival of motor neurons.The incidence of SMA is approximately 1 in 10,000 live births and it is the leading genetic cause of infant mortality. The most severe form of SMA is Type 1, a lethal genetic disorder characterized by rapid motor neuron loss and associated muscle deterioration, resulting in mortality or the need for permanent ventilation support by 24 months of age for more than 90 percent of patients if left untreated.
The efficacy of Zolgensma® in pediatric patients less than 2 years of age with SMA with bi-allelic mutations in the SMN1 gene was evaluated in STR1VE, an open-label, single-arm clinical trial (ongoing), and in START, an open-label, single-arm, ascending-dose clinical trial (completed). Patients experienced onset of clinical symptoms consistent with SMA before 6 months of age. All patients had genetically confirmed bi-allelic SMN1 gene deletions, 2 copies of the SMN2 gene, and absence of the c.859G>C modification in exon 7 of SMN2 gene (which predicts a milder phenotype). All patients had baseline anti-AAV9 antibody titers of <= 1:50, measured by ELISA. In both trials, Zolgensma® was delivered as a single-dose intravenous infusion.
Efficacy was established on the basis of survival, and achievement of developmental motor milestones such as sitting without support. Survival was defined as time from birth to either death or permanent ventilation. Permanent ventilation was defined as requiring invasive ventilation (tracheostomy), or respiratory assistance for 16 or more hours per day (including noninvasive ventilatory support) continuously for 14 or more days in the absence of an acute reversible illness, excluding perioperative ventilation. Efficacy was also supported by assessments of ventilator use, nutritional support and scores on the Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND). CHOP-INTEND is an assessment of motor skills in patients with infantile-onset SMA.
The ongoing clinical trial, STR1VE, enrolled 21 patients (10 male and 11 female) with infantile-onset SMA. Before treatment with Zolgensma, none of the 21 patients required non-invasive ventilator (NIV) support, and all patients could exclusively feed orally (i.e., no need for non-oral nutrition). The mean CHOP-INTEND score at baseline was 31.0 (range 18 to 47). All the patients received 1.1 × 10[1][4] vg/kg of Zolgensma. The mean age of the 21 patients at the time of treatment was 3.9 months (range 0.5 to 5.9 months).
As of the March 2019 data cutoff, 19 patients were alive without permanent ventilation (i.e., event-free survival) and were continuing in the trial, while one patient died at age 7.8 months due to disease progression, and one patient withdrew from the study at age 11.9 months. The 19 surviving patients who were continuing in the trial ranged in age from 9.4 to 18.5 months. By the data cutoff, 13 of the 19 patients continuing in the trial reached 14 months of age without permanent ventilation, one of the study's co-primary efficacy endpoints. In addition to survival, assessment of the other co-primary efficacy endpoint found that 10 of the 21 patients (47.6%) achieved the ability to sit without support for >= 30 seconds between 9.2 and 16.9 months of age (mean age was 12.1 months). Based on the natural history of the disease, patients who met the study entry criteria would not be expected to attain the ability to sit without support, and only approximately 25% of these patients would be expected to survive (i.e., being alive without permanent ventilation) beyond 14 months of age. In addition, 16 of the 19 patients had not required daily NIV use.
Comparison of the results of the ongoing clinical trial to available natural history data of patients with infantile-onset SMA provides primary evidence of the effectiveness of Zolgensma.
The completed clinical trial, START, enrolled 15 patients (6 male and 9 female) with infantile-onset SMA, 3 in a low-dose cohort and 12 in a high-dose cohort. At the time of treatment, the mean age of patients in the low-dose cohort was 6.3 months (range 5.9 to 7.2 months), and 3.4 months (range 0.9 to 7.9 months) in the high-dose cohort. The dosage received by patients in the low-dose cohort was approximately one-third of the dosage received by patients in the high-dose cohort. However, the precise dosages of Zolgensma received by patients in this completed clinical trial are unclear due to a change in the method of measuring Zolgensma concentration, and to decreases in the concentration of stored Zolgensma over time. The retrospectively-estimated dosage range in the high-dose cohort is approximately 1.1 × 10[1][4] to 1.4 × 10[1][4] vg/kg.
By 24 months following Zolgensma® infusion, one patient in the low-dose cohort met the endpoint of permanent ventilation; all 12 patients in the high-dose cohort were alive without permanent ventilation. None of the patients in the low-dose cohort were able to sit without support, or to stand or walk; in the high-dose cohort, 9 of the 12 patients (75.0%) were able to sit without support for >= 30 seconds, and 2 patients (16.7%) were able to stand and walk without assistance. Comparison of the results of the low-dose cohort to the results of the high-dose cohort shows a dose-response relationship that supports the effectiveness of Zolgensma®.
Limitation of Use:
The safety and effectiveness of repeat administration of Zolgensma have not been evaluated. The use of Zolgensma in patients with advanced SMA (e.g., complete paralysis of limbs, permanent ventilator-dependence) has not been evaluated.
Important Safety Information
Acute Serious Liver Injury
Acute serious liver injury and elevated aminotransferases can occur with Zolgensma. Patients with pre-existing liver impairment may be at higher risk. Prior to infusion, assess liver function of all patients by clinical examination and laboratory testing (e.g., hepatic aminotransferases [aspartate aminotransferase and alanine aminotransferase], total bilirubin and prothrombin time). Administer systemic corticosteroid to all patients before and after Zolgensma infusion. Continue to monitor liver function for at least 3 months after infusion.
Thrombocytopenia
Transient decreases in platelet counts, some of which met the criteria for thrombocytopenia, were observed at different time points after Zolgensma infusion. Monitor platelet counts before Zolgensma infusion and on a regular basis afterwards.
Elevated Troponin-I
Transient increases in cardiac troponin-I levels (up to 0.176 mcg/L) were observed following Zolgensma infusion in clinical trials. The clinical importance of these findings is not known. However, cardiac toxicity was observed in animal studies. Monitor troponin-I before Zolgensma infusion and on a regular basis for at least 3 months afterwards.
Adverse Reactions
The most commonly observed adverse reactions (incidence >=5%) were elevated aminotransferases and vomiting. Full prescribing information is available at https://www.avexis.com/content/pdf/prescribing_information.pdf
Source: Novartis
Read More
- Details
- ICNA
- News
- Hits: 1320
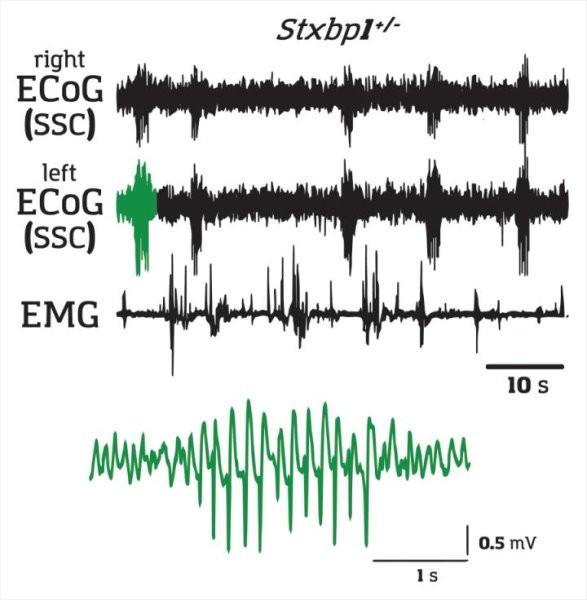
Kazuhiro Yamakawa and his team at the RIKEN Center for Brain Science (CBS) in Japan has shown that absence epilepsy can be triggered by impaired communication between two brain regions: the cortex and the striatum.
The researchers took STXBP1 and SCN2A genes created mice with one normal gene and one mutated gene -- a condition called haplodeficiency, which is different from a complete knockout. They showed that Spike Wave Discharges (SWD) can be blocked by drugs than inhibit neurons from exciting each other. The scientists injected a neuronal inhibitor into several brain regions hoping to find which ones were related to the seizures. They found three regions: somatosensory cortex, the thalamus, and a part of the striatum beneath the cortex.
It is generally believed that the thalamus and the somatosensory cortex are the primary sources for absence seizures. However Yamakawa and his group's experiments indicate that the critical trigger for absence seizures lie in the striatum.
After finding that injecting a neuron-exciting drug only into the striatal region of the model mice reliably induced SWDs, they created mice with mutations limited to only neurons in the somatosensory cortex that were connected to the striatum. These mice showed the same SWDs, meaning that absence seizures were triggered by faulty signals arriving in the striatum. An additional experiment showed that the problem arose because transmission specifically to fast-spiking interneurons in the striatum was too weak.
These findings potentially represent a paradigm shift for epilepsy research
Article:
Hiroyuki Miyamoto, Tetsuya Tatsukawa, Atsushi Shimohata, Tetsushi Yamagata, Toshimitsu Suzuki, Kenji Amano, Emi Mazaki, Matthieu Raveau, Ikuo Ogiwara, Atsuko Oba-Asaka, Takao K. Hensch, Shigeyoshi Itohara, Kenji Sakimura, Kenta Kobayashi, Kazuto Kobayashi, Kazuhiro Yamakawa. Impaired cortico-striatal excitatory transmission triggers epilepsy. Nature Communications, 2019; 10 (1) DOI: 10.1038/s41467-019-09954-9
Read More
- Details
- ICNA
- News
- Hits: 1338

Leonardo da Vinci produced some of the world’s most iconic art, but historical accounts of his work practices and behaviour show that he struggled to complete projects. Drawing on these accounts, Professor Catani lays out the evidence supporting his hypothesis that, as well as explaining his chronic procrastination, ADHD could have been a factor in Leonardo’s extraordinary creativity and achievements across the arts and sciences.
Professor Catani, from the Institute of Psychiatry, Psychology & Neuroscience at King’s, says: ‘While impossible to make a post-mortem diagnosis for someone who lived 500 years ago, I am confident that ADHD is the most convincing and scientifically plausible hypothesis to explain Leonardo’s difficulty in finishing his works. Historical records show Leonardo spent excessive time planning projects but lacked perseverance. ADHD could explain aspects of Leonardo’s temperament and his strange mercurial genius.’
ADHD is a behavioural disorder characterised by continuous procrastination, the inability to complete tasks, mind-wandering and a restlessness of the body and mind. While most commonly recognised in childhood, ADHD is increasingly being diagnosed among adults including university students and people with successful careers.
Leonardo’s difficulties with sticking to tasks were pervasive from childhood. Accounts from biographers and contemporaries show Leonardo was constantly on the go, often jumping from task to task. Like many of those suffering with ADHD, he slept very little and worked continuously night and day by alternating rapid cycles of short naps and time awake.
Alongside reports of erratic behaviour and incomplete projects from fellow artists and patrons, including Pope Leone X, there is indirect evidence to suggest that Leonardo’s brain was organised differently compared to average. He was left-handed and likely to be both dyslexic and have a dominance for language in the right-hand side of his brain, all of which are common among people with ADHD.
Perhaps the most distinctive and yet disruptive side of Leonardo’s mind was his voracious curiosity, which both propelled his creativity and also distracted him. Professor Catani suggests ADHD can have positive effects, for example mind-wandering can fuel creativity and originality. However, while beneficial in the initial stages of the creative process, the same traits can be a hindrance when interest shifts to something else.
Professor Catani, who specialises in treating neurodevelopmental conditions like autism and ADHD, says: ‘There is a prevailing misconception that ADHD is typical of misbehaving children with low intelligence, destined for a troubled life. On the contrary, most of the adults I see in my clinic report having been bright, intuitive children but develop symptoms of anxiety and depression later in life for having failed to achieve their potential.’
‘It is incredible that Leonardo considered himself as someone who had failed in life. I hope that the case of Leonardo shows that ADHD is not linked to low IQ or lack of creativity but rather the difficulty of capitalising on natural talents. I hope that Leonardo’s legacy can help us to change some of the stigma around ADHD.’
Source: King's College London
Article: Marco Catani, Paolo Mazzarello. Leonardo da Vinci: a genius driven to distraction. Brain, 2019; DOI: 10.1093/brain/awz131
Image credit:
Statue of Leonardo da Vinci.
Credit: © ArTo / Adobe Stock
Read More
- Details
- ICNA
- News
- Hits: 1154