Lenmeldy (atidarsagene autotemcel) has received approval from the U.S. Food and Drug Administration. This marks a significant milestone as it is the first gene therapy to be approved by the FDA for the treatment of children with pre-symptomatic late infantile, pre-symptomatic early juvenile or early symptomatic early juvenile metachromatic leukodystrophy (MLD).
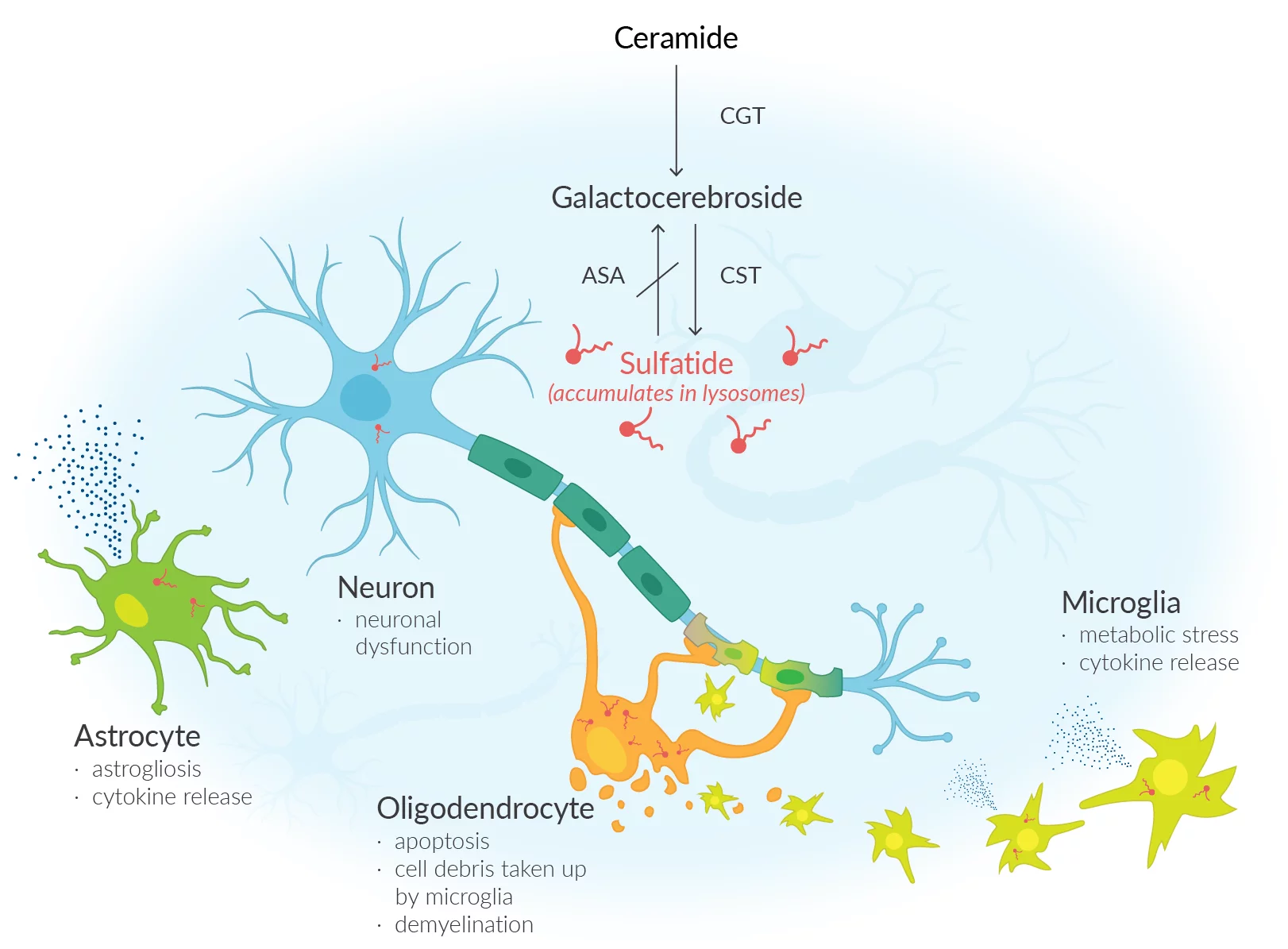
Metachromatic leukodystrophy a debilitating rare condition arises due to a lack of an enzyme known as arylsulfatase A (ARSA), resulting in an accumulation of sulfatides (fatty substances) within the cells. The accumulation of this substance results in damage to both the central and peripheral nervous systems, resulting in the decline of motor and cognitive abilities and ultimately premature mortality. Approximately one in every 40,000 individuals in the United States is affected by MLD. Unfortunately, MLD does not currently have a cure. The main approach to treatment involves providing support and managing symptoms.
Lenmeldy is a unique treatment that involves a personalized infusion of the patient's own hematopoietic (blood) stem cells. These cells have been genetically modified to include functional copies of the ARSA gene. The stem cells are obtained from the patient and modified by incorporating a functional copy of the ARSA gene. The modified stem cells are transplanted back into the patient, where they attach and multiply within the bone marrow. The modified stem cells provide the body with myeloid (immune) cells that generate the ARSA enzyme, aiding in the breakdown of sulfatides and potentially halting the advancement of MLD. Before treatment, patients are required to undergo high-dose chemotherapy, which involves the removal of cells from the bone marrow to make way for the modified cells in Lenmeldy.
The safety and effectiveness of Lenmeldy was evaluated using data from 37 children who participated in two single-arm, open-label clinical trials and an expanded access program. Children who underwent treatment with Lenmeldy were compared to a group of untreated children, allowing for a comparison of their progress over time. The main measure of effectiveness was the duration of time without severe motor impairment, which was defined as the period from birth until the first instance of losing the ability to move and sit without assistance, or until death. In children diagnosed with MLD, the administration of Lenmeldy has shown significant effectiveness in reducing the likelihood of severe motor impairment or mortality when compared to children who did not receive treatment. At 6 years of age, all children who received treatment with Lenmeldy were still alive, in contrast to only 58% of children in the natural history group. By the age of 5, a significant majority of children who received treatment were able to walk without any help. The majority of the children who received treatment showed normal language and performance IQ scores, a finding that has not been observed in children who did not receive treatment. Furthermore, young patients who exhibited pre-symptomatic early juvenile and early symptomatic early juvenile MLD experienced a decline in motor and/or cognitive function.
Some potential side effects of Lenmeldy include fever, low white blood cell count, mouth sores, respiratory infections, rash, medical line infections, viral infections, gastrointestinal infections, and enlarged liver.
Following the administration of Lenmeldy, it is important to closely observe patients for any changes in neutrophil counts and the potential for delayed platelet engraftment until engraftment has been successfully accomplished. There are potential risks associated with Lenmeldy treatment, including the possibility of blood clot formation or the development of encephalitis, a swelling of the brain tissues. Although there is a potential risk of blood cancer, it is important to note that no cases have been observed in patients who have undergone treatment with Lenmeldy. It is important for patients to undergo regular monitoring for hematologic malignancies, which includes an annual complete blood count (with differential). Integration site analysis should also be considered, as needed, for a minimum of 15 years following treatment.
- Details
- ICNA
- News
- Hits: 6153
 At least two powerful earthquakes struck in southern Turkey and northern Syria in the early hours of Feb. 6. At least 6,200 people have been killed with thousands more injured. The real death toll and impact of the disaster, however, remains to be seen as emergency services race to rescue families and children trapped beneath the rubble.
At least two powerful earthquakes struck in southern Turkey and northern Syria in the early hours of Feb. 6. At least 6,200 people have been killed with thousands more injured. The real death toll and impact of the disaster, however, remains to be seen as emergency services race to rescue families and children trapped beneath the rubble.
The ICNA stands in support of the children, their families, our colleagues and others who have been affected by this natural disaster. We will never forget the warm Turkish hospitality we experienced during our recent ICNA congress 2022 in Antalya from the members of the Turkish Child Neurology Association. In solidarity, we offer our support to our many friends and colleagues and their families as well as to the vulnerable children they care for at this very sad and critical time of suffering and hope for the minimum of loss.
- Details
- ICNA
- News
- Hits: 15416
Read more: Support the children and their families impacted by the Earthquake in Türkiye & Syria

 I am deeply saddened to learn that Michael V Johnston MD. Professor Emeritus of Pediatrics and Neurology at the Johns Hopkins University School of Medicine and former Chief Medical Officer and Executive Vice President at the Kennedy Krieger Institute, Baltimore, passed away on July 30, 2022. Dr Johnston (Mike) was a very active and cherished member of the ICNA and served on the executive board for two terms. He was a brilliant academician and researcher and his seminal work on the role of glutamate and excitotoxicity in hypoxic ischaemic injury and its effects on the developing brain is well known internationally. A great mentor, he was very supportive and patient, and was admired by all his mentees. He collaborated with clinicians and researchers and continued to treat patients with complex neurological disorders.
I am deeply saddened to learn that Michael V Johnston MD. Professor Emeritus of Pediatrics and Neurology at the Johns Hopkins University School of Medicine and former Chief Medical Officer and Executive Vice President at the Kennedy Krieger Institute, Baltimore, passed away on July 30, 2022. Dr Johnston (Mike) was a very active and cherished member of the ICNA and served on the executive board for two terms. He was a brilliant academician and researcher and his seminal work on the role of glutamate and excitotoxicity in hypoxic ischaemic injury and its effects on the developing brain is well known internationally. A great mentor, he was very supportive and patient, and was admired by all his mentees. He collaborated with clinicians and researchers and continued to treat patients with complex neurological disorders.
Along with his friend and colleague, Dr Gary Goldstein, he spearheaded the Kennedy Krieger Institute for 31 years, into an Institution known for cutting edge research and for providing state-of-the-art management to children with neuro-developmental disorders. He published hundreds of research papers and gave numerous invited lectures across the world. He received several awards and honours including the Frank Ford Lecture award of the ICNA (2016), the Blum/ Moser Endowed Chair for Paediatric Neurology at the Kennedy Krieger Institute and the Bernard Sachs Award from the Child Neurology Society.
I had the privilege of being one of the many people whose lives he touched. I was closely associated with him since 1991 when I worked with him at the Kennedy Krieger Institute. Subsequently he visited our Institution -The Post Graduate Institute for Medical Education and Research Chandigarh, India, as a visiting Professor and an invited speaker periodically. He had qualities of the head and heart both! Despite his several professional accomplishments, he was such a humble, caring, and friendly person and a truly devoted family man. Both, he, and his wife Sally, loved music, hosted many friends, and went out of their way to help people. He is survived by his loving wife Sally, three sons and their wives, and eight lovely grandchildren. Mike will be missed not only by the ICNA community, but by Paediatric Neurologists all over the world.
Pratibha Singhi
Secretary and President Elect
ICNA
Also see:
Michael VanDoren Johnston, M.D. Obituary
Michael V. Johnston, M.D., Professor Emeritus of Pediatrics and Neurology at Johns Hopkins University School of Medicine and former Chief Medical Officer and Executive Vice President at the Kennedy Krieger Institute, passed away peacefully at home in Baltimore on July 30, 2022. The cause was pancreatic cancer.
Dr. Johnston (“Mike”) was born January 30, 1946 in Pittsburgh, and raised in Christiana, a small town outside of Lancaster, Pennsylvania. His father, Eugene, was a physician and his mother, Naomi, was a nurse. Together Mike’s parents ran a general practice out of their home. From a young age, Mike wanted to be a doctor and would accompany his father on house calls to patients living on the farms that surround Christiana. Many of his father’s patients were Amish, and Mike helped build a hitching post in front of the home office to allow them to secure their horses and buggies during visits.
After graduating as Valedictorian from Octorara High School in 1963, Mike went on to Franklin and Marshall College in Lancaster. It was there that he met the love of his life and future wife, Susan (“Sally”) Johnston, who had traveled to Franklin and Marshall from Chatham College in Pittsburgh as part of a visiting choir to sing the opera, Dido and Aeneas. Mike and Sally liked to say that their love of music brought them together, and Mike was a lifelong lover of opera music. Mike and Sally were married in 1968 in Pittsburgh where Mike was attending medical school at the University of Pittsburgh School of Medicine. Dr. Johnston graduated from medical school in 1971, first in his class, and he and Sally then moved to Baltimore for his internship and residency in Pediatrics at The Johns Hopkins University School of Medicine. After completing his pediatrics residency, Dr. Johnston was drafted into the U.S. Army and served two years working for the U.S. Army Office of the Surgeon General in Washington, D.C. He then returned to Johns Hopkins in 1976 for residency in Neurology. Dr. Johnston was drawn to pediatric neurology because he was fascinated by the brain and saw enormous potential in the field for new discoveries. It was during this time that he worked in the laboratory of Dr. Joseph T. Coyle, where their work resulted in the discovery of the role of the nucleus basalis in the developing brain. In 1980 Dr. Johnston started his first faculty position at the University of Michigan Medical School. During his time in Ann Arbor, Dr. Johnston conducted pivotal research into the role of glutamate in hypoxic ischemic brain injury and its effects on the developing brain. He was rapidly promoted, becoming a full Professor of Pediatrics & Neurology at the University of Michigan in 1987.
In 1988 Dr. Johnston returned to Baltimore to help lead the Kennedy Krieger Institute with his long-time friend and colleague, Dr. Gary Goldstein. This was his dream job. Kennedy Krieger allowed Dr. Johnston the opportunity to continue his research and collaborate with brilliant clinicians and researchers, while also continuing to treat patients. His patients often had rare and complex neurological disorders, but Dr. Johnston saw potential. With his knowledge and compassion, he endeavored to bring hope to these children and their families. Over 31 years at Kennedy Krieger Dr. Johnston helped grow the institution into a world leader in research and clinical care of children with neurologic and developmental disorders. Over his career Dr. Johnston published hundreds of peer-reviewed articles, chapters, and textbooks, and lectured around the world. He also mentored countless numbers of young researchers and clinicians and was generous with his time. In recognition of his achievements, he served on the American Board of Psychiatry and Neurology (ABPN) and served as the President of ABPN in 2007. He in addition received numerous honors and awards including the Bernard Sachs Award from the Child Neurology Society (2008), the Blum/Moser Endowed Chair for Pediatric Neurology at Kennedy Krieger Institute (2009), and the Frank Ford Lecture Award from the International Child Neurology Association (ICNA) (2015).
Aside from his professional career, Dr. Johnston was devoted to his family. He cherished every moment he was able to spend with his family on vacation in Rangeley, Maine, where he had vacationed with his family as a boy. Sally was a native of Yarmouth, Maine and from the time their three boys were little, Sally and Mike spent nearly every summer vacation in Maine, along with extended family. Dr. Johnston is survived by his loving wife of 54 years, Sally Johnston, his three sons, Peter, Jamie, and Joe, his daughters-in-law Cecilia, Kristin, and Jennie, and—the light of his life—his eight grandchildren, Elizabeth, Michael, Andrew, Ryan, Caroline, Eli, Scarlett, and Liam. His two siblings, David Johnston, M.D. and Marcia Johnson, predeceased him.
A Memorial Service for Dr. Johnston will be held on Saturday, August 20 at 11 a.m. at the Second Presbyterian Church, 4200 St Paul St, Baltimore, MD 21218. In lieu of flowers, memorial contributions may be made in his name to Kennedy Krieger Institute at www.KennedyKrieger.org/Tributes or direct your gift to the Office of Philanthropy, Kennedy Krieger Foundation, 707 N. Broadway, Baltimore, MD 21205.
- Details
- ICNA
- News
- Hits: 9180
 It is with great sadness we share that Alcy Torres Catefort Sr, MD, father of our esteemed colleague Dr. R. Alcy Torres, a global health leader himself, died January 9, 2022, at home. He was 84.
It is with great sadness we share that Alcy Torres Catefort Sr, MD, father of our esteemed colleague Dr. R. Alcy Torres, a global health leader himself, died January 9, 2022, at home. He was 84.
Born in Ecuador, Dr. Torres received his MD from Universidad Central del Ecuador. He completed his residency in Pediatrics at Children’s Hospital of Paris (Necker-Enfants Malades) in 1968 and obtained additional training on Neonatology at the Mexican Institute of Social Security (IMSS) in Mexico. Dr. Torres’s career began with his service long before becoming a pediatrician. He was an intern at Hospital Baca-Ortiz (Children’s Hospital of Quito), where he acquired excellent experience for several years which became the base of his pediatric knowledge. After becoming a pediatrician, he joined the Carlos Andrade Marin Hospital (HCAM) in Quito, Ecuador, in 1972, where he subsequently became the founder and Director of the Pediatric Emergency Department, Pediatric Critical Care, and General Pediatrics.
Although Dr. Torres continued as Director of Pediatrics, he dedicated himself to Pediatric Neurology, a specialty that he practiced until his retirement in 2010 at the age of 72. During his tenure, the Pediatric Service became a Department with the highest complexity level of care for pediatric referrals nationwide. When the social security law extended health coverage to workers' children and native Ecuadorians, Dr. Torres saw the need to expand the Pediatric Ward, and he developed a Pediatric Specialties Ward. He also developed a hospitality program that offered hospitality and food to families coming from long distances and requiring lengthy hospitalizations due to malaria, complicated meningitis, trauma, and cancer.
From his early years in medical school, Dr. Torres became involved in teaching, as Instructor of Anatomy, and after his graduation, he joined the Faculty of the Universidad Central del Ecuador, School of Medicine, in the area of Pediatrics. He was asked to develop the Pediatric Neurology program, the first in the country and shortly after, he wrote the Pediatric Neurology book, known as the “Red Book.” He quickly moved up through the academic ladder to become Professor of Pediatrics and Chairman of the Department of Pediatrics.
Dr. Torres’s scientific contributions and research interests as a pediatrician were to develop an alternative method for infant rehydration, gut repair, and early feeding with the “Soup de Carrot.” In the field of Pediatric Neurology, he focused on the study of neonatal encephalopathy, with several publications like the study of 1100 newborn babies with hypoxic ischemic encephalopathy, later included in his textbook of Pediatric Neurology Emergencies. In addition, Dr. Torres authored many publications during his scientific prolific career and several leading editions of his books on Pediatric Neurology.
Dr. Torres was greatly appreciated by his students and staff in general. His teaching style reached their hearts and was characterized by motivating them to improve. Besides scientific teaching, he shared his experiences of living in France and the ways to overcome the obstacles that life presents us all. Throughout his career, Dr. Torres received countless honors and accolades for his accomplishments and innovative program development. He initiated and oversaw programs to improve health outcomes for children with dehydration and hypoxic-ischemic injury and helped establish clinics providing specialized services to Ecuadorian minorities. Dr. Torres was a lifelong champion of Pediatric Emergency and Pediatric Neurology. In addition, he volunteered for more than 30 years caring for sick orphan children at Hogar del Nino, San Vicente de Paul, in the south of Quito, an area of extreme poverty.
Dr. Torres was a seminal force in academic medicine at the local, national, and international levels. He was President of the Ecuadorian Pediatric Society, President of the Academia Iberoamericana de Neurologia Pediatric Congress, member of the French Pediatric Society, and member of the Asociacion Latinoamericana de Pediatria, Executive Board in Neurology. He met AAP President Dr. Errol Alden in Rio de Janeiro during an IPA meeting to discuss how to improve medical education in our hemisphere, which led to formal education on NALS and later on PALS in many countries in Latin America. Dr. Torres’s guidance and passionate support for his students, trainees, and junior faculty helped mold countless generations of pediatricians in Ecuador. In each domain of service, he insisted on excellence and purpose, always demanding attention to the health and dreams of the neediest among us.
Dr. Torres had many and varied interests beyond medicine. He had a deep knowledge and passion for reading and music. He enjoyed traveling to the mountains and spending time with friends and family. He and his beloved wife Gladys Guerrero had three children, Alcy, Gladys, and Edmundo, all physicians with specialties in Pediatric Neurology, Ophthalmology, and Internal Medicine, respectively.
Dr. Torres will be remembered for his love for his family and friends, for his warmth and good humor, for his unusual combination of humility and dignity, for his unfailing curiosity in all things, and always, for his sense of style. The services were private. A celebration of Dr. Torres’s life will be held at a later date. Our sincere condolences to Dr. Torres’s family, friends, and colleagues and, particularly, to Alcy, his older son, who follows in his father’s steps in Boston and is an esteemed member of the ICNA.
Originally published in the AAP Section of Global Health Newsletter
- Details
- ICNA
- News
- Hits: 7818

- Details
- ICNA
- News
- Hits: 9147
Read more: A Child Without a Shadow: A Memoir of a Holocaust Survivor and a World Famous Doctor