 The ICNA is happy to announce an educational program to be held in Pune, India on January 19-20, 2013 in collaboration with Pune Neurological Society.
The ICNA is happy to announce an educational program to be held in Pune, India on January 19-20, 2013 in collaboration with Pune Neurological Society.
The ICNA will be represented by Doctors Harry Chugani, Banu Anlar, Linda De Meirleir, Peter Camfield, Carol Camfield.
The local organizer is Dr Nandan Yardi, K.E.M. Hospital Research Centre, Pune
Read More
- Details
- ICNA
- News
- Hits: 594
The 48-week results from the ongoing Phase IIb clinical trial of eteplirsen for the treatment of Duchenne muscular dystrophy (DMD)has been announced. Eteplirsen is an exon-skipping compound that addresses one of the underlying genetic defects in Duchenne muscular dystrophy.
At 48 weeks eteplirsen demonstrated a significant and unprecedented clinical benefit on the primary clinical outcome measure, the 6-minute walk test, and met the primary efficacy endpoint of the study, an increase in novel dystrophin. The results represent the potential medical breakthrough that eteplirsen represents for the treatment of DMD.
Although only about 13% of boys with Duchenne muscular dystrophy have the specific mutation targeted by eteplirsen, the implications for all Duchenne muscular dystrophy patients with related genetic mutations are clearly evident.
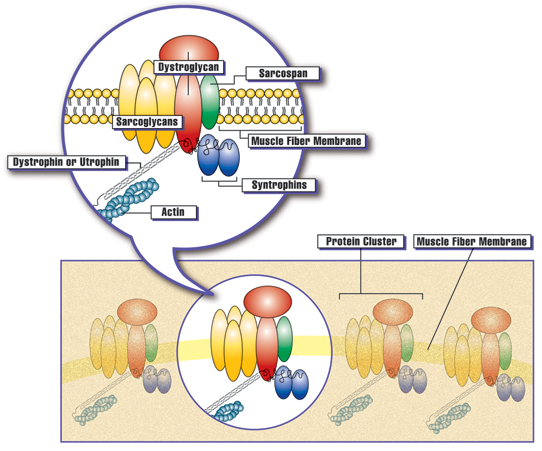 Clusters of proteins are spaced at intervals along the membrane of each muscle fiber. The presence of dystrophin, or the related protein utrophin, appears to be necessary for the other proteins in the cluster to assemble at the membrane. Courtesy: Muscular Dystrophy Association
Clusters of proteins are spaced at intervals along the membrane of each muscle fiber. The presence of dystrophin, or the related protein utrophin, appears to be necessary for the other proteins in the cluster to assemble at the membrane. Courtesy: Muscular Dystrophy Association
Trial Results
Eteplirsen administered once weekly at either 30mg/kg or 50mg/kg for 48 weeks (n=8) resulted in a statistically significant increase in dystrophin-positive fibers of 47.0% of normal.
The placebo/delayed treatment cohort, which received 24 weeks of eteplirsen at either 30mg/kg or 50mg/kg following 24 weeks of placebo (n=4), produced a statistically significant increase in dystrophin-positive fibers of 38.3% of normal.
In addition, eteplirsen administered once weekly at 50mg/kg over 48 weeks resulted in a 89.4 meter benefit compared to patients in our placebo/delayed treatment arm, those patients who received placebo for 24 weeks followed by 24 weeks of treatment with eteplirsen in the open-label extension of the study.
Upon evaluating all study participants through 48 weeks, no treatment-related adverse events which further demonstrate the highly favorable safety profile of eteplirsen.
An abstract describing the results from this Phase IIb extension study has been accepted as part of the World Muscle Society (WMS) Congress's Late-Breaking Science program in Perth, Australia during October 9 to October 13, 2012.
Principal investigator, Jerry R. Mendell, M.D. of Nationwide Children's Hospital, will present the data "Results at 48 Weeks of a Phase IIb Extension Study of the Exon-Skipping Drug Eteplirsen in Patients with Duchenne muscular dystrophy (DMD)" at the World Muscle Society (WMS) Congress's Late-Breaking Science program in Perth, Australia on October 13 at 4:00 p.m. WST UTC +8 hours/4:00 a.m. EDT.
Summary of Dystrophin: Eteplirsen-Treated Patients in All Dose Groups through Week 48*
| Treatment Arm | Mean Change from Baseline in % Dystrophin-Positive Fibers | p-value | |||
| Eterplirsen (both doses): 48 wks of Tx (n=8) | 47.0 | ≤0.001 | |||
| Eteplirsen 50 mg/kg (n=4) | 41.7 | ≤0.008 | |||
| Eteplirsen 30 mg/kg (n=4) | 52.1 | ≤0.001 | |||
| Placebo/Delayed Tx: 24 wks of Tx (n=4) | 38.3 | ≤0.009 | |||
| Placebo/50 mg/kg Delayed-Tx (n=2) | 42.9 | ns | |||
| Placebo/30 mg/kg Delayed-Tx (n=2) | 34.2 | ns | |||
* Values based on Immunofluorescence using anti-dystrophin antibody MANDYS106
Modified Intent-to-Treat (mITT)
The 6MWT results were further analyzed using the mITT population which excluded two patients who were randomized to the 30 mg/kg weekly eteplirsen cohort who showed signs of rapid disease progression within weeks after enrollment and were unable to perform measures of ambulation beyond 24 weeks. This mITT population consisted of 10 patients (4 eteplirsen-treated patients receiving 50 mg/kg weekly, 2 eteplirsen-treated patients receiving 30 mg/kg weekly, and 4 placebo/delayed-treatment patients).
Summary of 6MWT: Eteplirsen versus Placebo/Delayed-Treatment to Week 48*
| Treatment Arm | Mean Change from Baseline in 6MWT (meters) | Estimated Treatment Effect (Eteplirsen minus Placebo/Delayed-Tx) | p-value | |||
| Placebo/Delayed-Tx (n=4) | -60.3 | |||||
| Eteplirsen 50 mg/kg (n=4) | +27.1 | 87.4 m | ≤0.001 | |||
| Eteplirsen Both Doses (n=6) | +7.3 | 67.3 m | ≤0.001 | |||
| Eteplirsen 30 mg/kg (n=2) | -31.5 | 28.8 m | ns | |||
*Note: Analysis based on Mixed Model Repeated Measures test
Summary of Additional Sub-Group Analyses at Week 48*
| Subset | Mean 6MWT Change from Baseline (meters) | Estimated Treatment Benefit (Eteplirsen minus Placebo/delayed-Tx) | p-value | |||
| Placebo/delayed Tx: < 9.5 yrs at baseline (n=2; mean=7.6 yrs) |
-42.3 | 58.9 m | ≤0.038 | |||
| Eteplirsen: < 9.5 yrs at baseline (n=3; mean=8.4 yrs) |
+16.5 | |||||
| Placebo/delayed Tx: ≥9.5 yrs at baseline (n=2; mean=10.1 yrs) |
-63.5 | 52.1 m | ns | |||
| Eteplirsen: ≥9.5 yrs at baseline (n=3; mean=10.4 yrs) |
-11.3 | |||||
| Placebo/delayed Tx: Higher 6MWT baseline (n=2; mean=422m) |
-53.5 | 93.8 m | ≤0.001 | |||
| Eteplirsen: Higher 6MWT baseline (n=3; mean=424m) |
+40.3 | |||||
| Placebo/delayed Tx: Lower 6MWT baseline (n=2; mean=367m) |
-65.8 | 39.6 m | ns | |||
| Eteplirsen: Lower 6MWT baseline (n=3; mean=375m) |
-26.2 | |||||
| Placebo/delayed Tx: Genotype 49-50 deletion (n=3; age mean=9.2 yrs, 6MWT BL mean=397m) |
-69.0 | 83.4 m | ≤0.001 | |||
| Eteplirsen: Genotype 49-50 deletion (n=2; age mean=9.1 yrs, 6MWT BL mean=383m) |
+14.4 | |||||
* Note: Analysis based on Mixed Model Repeated Measures test
About Study 201 and Study 202 (Phase IIb Eteplirsen Study)
Study 4658-US-201 was conducted at Nationwide Children's Hospital in Columbus, Ohio. Twelve boys meeting the inclusion criteria being between 7 and 13 years of age with appropriate deletions of the dystrophin gene that confirm eligibility for treatment with an exon-51 skipping drug, received double-blind IV infusions of placebo (n=4), 30 mg/kg of eteplirsen (n=4), or 50 mg/kg of eteplirsen once weekly for 24 weeks (n=4). Muscle biopsies for evaluation of dystrophin were obtained at baseline for all subjects, and after 12 weeks for patients in the 50 mg/kg cohort and after 24 weeks for patients in the 30 mg/kg cohort. Two placebo patients were randomized to the 30 mg/kg cohort and two placebo patients were randomized to the 50 mg/kg cohort. This study design allowed to investigate the relationship of dose and duration of eteplirsen treatment on the production of dystrophin over the course of the 24-week study.
Study 4658-US-202 is the extension study to 201 and continues to assess the long-term safety and efficacy of open-label eteplirsen. The four placebo patients were rolled over to open-label eteplirsen at week 24, with six patients on 30 mgs/kg, and six patients on 50 mgs/kg. Third biopsies occurred at 48 weeks in the original study 201 treated patients, and at 24 weeks, the same time point, in the original placebo patients. 6MWT was performed at 32 weeks, 36 weeks, 48 weeks and will continue to be performed every 12 weeks going forward.
About Dystrophin
Dystrophin, a large structural protein, is critical to the stability of myofiber membranes in skeletal, diaphragmatic and cardiac muscle, protecting muscle fibers from contraction-induced damage. Loss of functional dystrophin destabilizes the dystroglycan protein complex, impairing its localization to the muscle membrane, and compromising the integrity of the membrane structure. The absence of functional dystrophin results in muscle membrane breakdown with muscle fibers being replaced by adipose and fibrotic tissue.
Eteplirsen
Eteplirsen uses phosphorodiamidate morpholino oligomer (PMO)-based chemistry and proprietary exon-skipping technology to skip exon 51 of the dystrophin gene enabling the repair of specific genetic mutations that affect approximately 13 percent of the total DMD population. By skipping exon 51, eteplirsen may restore the gene's ability to make a shorter, but still functional, form of dystrophin from messenger RNA, or mRNA. Promoting the synthesis of a truncated dystrophin protein is intended to improve, stabilize or significantly slow the disease process and prolong and improve the quality of life for patients with DMD.
About the 6-Minute Walk Test
The 6-minute walk test (6MWT) was developed as an integrated assessment of cardiac, respiratory, circulatory, and muscular capacity (American Thoracic Society 2002) for use in clinical trials of various cardiac and pulmonary conditions.
In recent years the 6MWT has been adapted to evaluate functional capacity in neuromuscular diseases and has served as the basis for regulatory approval of a number of drugs for rare diseases, with mean changes in the 6MWT ranging from 28 to 44 meters (Rubin 2002, Wraith 2004, Muenzer 2006).
Additionally, published data from longitudinal natural history studies assessing dystrophinopathy, a disease continuum comprised of DMD and Becker muscular dystrophy, support the utility of the 6MWT as a clinically meaningful endpoint (McDonald C, et al, Muscle & Nerve, December 2010) in DMD.
These data show that boys with DMD experience a significant decline in walking ability compared to healthy boys over one year, suggesting that slowing the loss of walking ability is a major treatment goal.
About the Statistical Methodology
The Mixed Model Repeated Measures (MMRM) test was used for all statistical analyses of the 6MWT results, including for all subgroups. Analysis of Covariance (ANCOVA) for ranked data was used when the assumptions of normality of the dependent variable (the change in 6MWT distance from baseline) were violated.
The inclusion of the two patients with extreme scores due to rapid progression in the ITT population (n=12) resulted in a violation of the normality assumptions of the Change from Baseline in 6MWT data, and thus required the use of ANCOVA for ranked data.
The exclusion of these two patients from the mITT population (n=10) resulted in the 6MWT data becoming normally distributed and the MMRM statistics exhibiting much improved residuals and fit statistics as compared to the ITT population.
As such, the estimated mean values and their associated p-values for the mITT population were slightly different from those for the ITT population.
Sources:
SAREPTA
Action Duchenne
Other resources:
SAREPTA will hold a conference call and broadcast a slide show today at 8:00 a.m. EDT (5:00 a.m. PDT) to discuss these results. The audio conference call may be accessed by dialing 866.356.3093 for domestic callers and 617.597.5381 for international callers. The passcode for the call is 93880948. Please specify to the operator that you would like to join the "Sarepta Therapeutics 48-Week Results Call." To view the slide show while using the audio dial-in please go to the events section of Sarepta's website at www.sareptatherapeutics.com. The call and slide show will also be webcast live under the events section and will be archived there following the call for 90 days. Please connect to Sarepta's website several minutes prior to the start of the broadcast to ensure adequate time for any software download that may be necessary. An audio replay will be available through October 10, 2012 by calling 888.286.8010 or 617.801.6888 and entering access code 67898748.
Read More
- Details
- ICNA
- News
- Hits: 830
 In an article published in the Lancet on September 27, Professor Newton and Professor Hector Garcia, both Wellcome Trust Senior Research Fellows describes the burden of epilepsy in in poor areas of the world.
In an article published in the Lancet on September 27, Professor Newton and Professor Hector Garcia, both Wellcome Trust Senior Research Fellows describes the burden of epilepsy in in poor areas of the world.
They conducted a comprehensive review of academic articles about epilepsy in developing countries in order to piece together a picture of the burden of the disease in poorer parts of the world.
The burden of epilepsy in low-income countries is more than twice that found in high-income countries, probably because the incidence of risk factors for eg head trauma, complications during childbirth, and parasite infections such as pork tapeworm (neurocysticercosis), and river blindness (onchocerciasis) is higher.
The researchers call for greater recognition from international and national health agencies to address the management of epilepsy in the developing world.
Epilepsy is associated with substantial stigma in low-income countries, which acts as a barrier to patients accessing biomedical treatment and becoming integrated within society. Seizures can be controlled by inexpensive antiepileptic drugs, but the supply and quality of these drugs can be erratic in poor areas. Prof. Charles Newton
Prof. Charles Newton
The treatment gap for epilepsy is high (>60%) in deprived areas, but this could be reduced with low-cost interventions. Despite being one of the most cost-effective disorders to treat, there are twice as many people living with epilepsy in low- and lower-middle-income countries than higher income nations and more than 60% of those affected in these regions are not accessing any appropriate treatment.
Epilepsy needs to be brought into the agenda of non-communicable diseases. It was not mentioned in the UN General Assembly Meeting in New York to address the global burden of non-communicable diseases, and yet it represents a substantial burden of ill health- Professor Charles Newton
"Sadly, adequate facilities for diagnosis, treatment and on-going management of epilepsy are virtually non-existent in many of the world's poorest regions. Many people with epilepsy or their families do not even know that they have a disorder that can be controlled with biomedical treatment, so it is vitally important that awareness is raised and medical care improved in these regions," added Professor Newton. who works in the Wellcome Trust programmes in Tanzania and Kenya
The authors call for greater recognition from international and national health agencies to address the management of epilepsy in the developing world.
Journal article:
Charles R Newton, Hector H Garcia, Epilepsy in poor regions of the world, The Lancet, Volume 380, Issue 9848, 29 September–5 October 2012, Pages 1193-1201, ISSN 0140-6736, 10.1016/S0140-6736(12)61381-6. (http://www.sciencedirect.com/science/article/pii/S0140673612613816)
Read More
- Details
- ICNA
- News
- Hits: 803
 Children suffering from prolonged, acute, convulsive seizures may not always receive timely rescue medication in schools and other community settings as intended by their specialist physician, according to the first findings of the PERFECT[1] Initiative. The results were presented as part of a symposium at the ILAE's 10th European Congress on Epileptology (ECE), in London. The Steering Committee for The PERFECTTM Initiative, which comprises a group of leading clinical epilepsy specialists from six countries across Europe, also highlight discrepancies in comprehensive European guidelines and legal frameworks that ensure children with prolonged, acute, convulsive seizures are treated quickly whether in hospital or in the community, and recommend specific training on rescue medication for all those responsible for the child.[i]
Children suffering from prolonged, acute, convulsive seizures may not always receive timely rescue medication in schools and other community settings as intended by their specialist physician, according to the first findings of the PERFECT[1] Initiative. The results were presented as part of a symposium at the ILAE's 10th European Congress on Epileptology (ECE), in London. The Steering Committee for The PERFECTTM Initiative, which comprises a group of leading clinical epilepsy specialists from six countries across Europe, also highlight discrepancies in comprehensive European guidelines and legal frameworks that ensure children with prolonged, acute, convulsive seizures are treated quickly whether in hospital or in the community, and recommend specific training on rescue medication for all those responsible for the child.[i]
Prolonged, acute, convulsive seizures can pose a significant health threat in children with epilepsy, a neurological disorder affecting nearly one million children and adolescents in Europe.[ii] Evidence suggests that treatment should be given immediately if a seizure persists longer than 5 minutes after onset.[iii] However, in the case of schools, despite the fact that many children are prescribed rescue medication by their doctors, teachers often opt not to administer seizure rescue medication unless specific training or provision has been made, typically via the school nurse. Instead an ambulance may be called, causing possible delays in seizure treatment.[iv]
"The PERFECT Initiative is the first to investigate the discrepancies that often exist in European countries between policy and practice in the treatment of prolonged, acute, convulsive seizures in children," said Prof J. Helen Cross, UCL Institute of Child Health, Great Ormond Street Hospital for Children and Young Epilepsy. "We found that the differences in clear guidance, awareness and education around the use of rescue medication for treating seizures in children living with epilepsy, ultimately create a shortfall in care that we, as clinicians, intend that they receive, whether in hospital or away from it."
This first phase of the PERFECT Initiative was designed to examine existing treatment guidelines and legal frameworks and policies for treating prolonged, acute, convulsive seizures in the community, in six European countries (France, Germany, Italy, Spain, Sweden and the UK). The authors found that, while guidelines were effective for in-hospital treatment of prolonged, acute, convulsive seizures, the picture was often different in the community. In many cases, rescue medication consists of intra-rectal diazepam, which can be considered socially inappropriate to administer in the community setting. Whether a child receives rescue medication at school depends primarily on the availability of staff willing to accept responsibility for administering the treatment.1 Key recommendations from the PERFECT Initiative Steering Committee include:1
Establishing clear links between the treating physician, families and the child's day-to-day community environment (e.g. schools), allowing for better provision of information on epilepsy and training on seizure intervention for all those individuals responsible for the child
New and revised comprehensive guidelines to ensure children with prolonged, acute, convulsive seizures are treated according to the treatment plan set by their physician, wherever the seizure occurs
Individualised treatment plans for every child are established between the treating physician and the family/carers concerned, to help ensure the best possible standards of care for the child away from the hospital setting.
ViroPharma Incorporated (Nasdaq: VPHM), as part of its commitment to help create better care for children with prolonged, acute, convulsive seizures, has organised and funded the PERFECT Initiative in collaboration with a group of leading clinical epilepsy specialists. Vanessa Newman, Associate Director Medical Affairs, Europe, at ViroPharma said: "The PERFECT Initiative was designed to investigate any possible shortfalls in the current approach in Europe to the management of prolonged, acute, convulsive, seizures in children treated in the community with rescue medication. We believe that we can help improve care for children at risk of seizures by supporting collaboration between key stakeholders, identifying opportunities for social integration, and facilitating better education at all levels. We are proud to have initiated PERFECT and look forward to helping support the improvement in care for children with epilepsy that The Steering Committee recommends."
The second two phases of the PERFECT Initiative include a survey of physicians and nurses who treat children with prolonged, acute, convulsive seizures, followed by a survey of children and their parents regarding their treatment, care, guidance and quality of life. Results of these phases are due to be published in 2013.
About the PERFECT Initiative
ViroPharma, as part of its commitment to help create better care for children with prolonged, acute, convulsive seizures, has organised and funded the PERFECT (Practices in Emergency and Rescue medication for Epilepsy managed with Community administered Therapy) Initiative in collaboration with a Steering Committee group of leading clinical epilepsy specialists. The Initiative aims to document and communicate first of its kind data on the impact of conflict of policy and practice in the treatment of prolonged, acute, convulsive seizures.
The PERFECT Initiative is designed to facilitate collaboration between key stakeholders, identifying opportunities for social integration around children experiencing breakthrough seizures, and supporting means of better education of the condition at the community level. It consists of three phases, a review and comparison of policy and real-world practice, a survey of physicians and nurses who treat children with prolonged, acute, convulsive seizures, and a survey of children and their carers relating to their experiences of breakthrough seizures.
References:
[1] Practices in Emergency and Rescue medication For Epilepsy managed with Community administered Therapy
[i] Wait S, et al. The administration of rescue medication to children with prolonged acute convulsive seizures in the community: what happens in practice? Eur J Paediatr Neurol. 2012. doi:10.1016/j.ejpn.2012.07.002. Published early online.
[ii] Epilepsy in the WHO European Region: Fostering Epilepsy Care in Europe. 2010. Available at: http://www.ibe-epilepsy.org/downloads/EURO%20Report%20160510.pdf. Last accessed July 2012.
[iii] Lagae L. The treatment of acute convulsive seizures in children. Eur J Pediatr 2011;170:413-8. [iv] Kriel RL, et al. Home use of rectal diazepam for cluster and acute prolonged seizures: efficacy, adverse reactions, quality of life, and cost analysis. Pediatr Neurol 1991;7:13-17.
[v] Ekinci O, et al. Depression and anxiety in children and adolescents with epilepsy: Prevalence, risk factors, and treatment. Epilepsy Behav 2009;14:8-18.
Read More
- Details
- ICNA
- News
- Hits: 755
 The EFNS-ENS consensus recommendations for the usage of PCR technology for the diagnosis of infections of the nervous system have been published. A summary of the guideline is given below.
The EFNS-ENS consensus recommendations for the usage of PCR technology for the diagnosis of infections of the nervous system have been published. A summary of the guideline is given below.
Polymerase chain reaction (PCR) is a simple and rapid, easy- to- use approach to diagnose infections by amplifying nucleic acids. The reliability of PCR technology for the diagnosis of neurological infections is dependant on the type of pathogen
The PCR involves the in vitro enzymatic synthesis of millions of copies of a specific DNA segment.
1. The reaction is based on the annealing and extension of two oligonucleotide primers that flank the target region in duplex DNA
2. After denaturation of the DNA, each primer hybridizes to one of the two separated strands such that extension from each 3′ hydroxyl end is directed toward the other.
3. The annealed primers are then extended on the template strand with a DNA polymerase.
These three steps (denaturation, primer binding, and DNA synthesis) represent a single PCR cycle. Consequently, repeated cycles of denaturation, primer annealing, and primer extension result in the exponential accumulation of a discrete fragment whose termini are defined by the 5′ ends of the primers.
The length of the products generated during the PCR is equal to the sum of the lengths of the two primers plus the distance in the target DNA between the primers. PCR can amplify double or single-stranded DNA, and with the reverse transcription of RNA into a cDNA copy, RNA can also serve as a target.
Quantitative PCR, uses precision optics and DNA-binding fluorescent dyes or fluorescent labels to monitor amplification in real-time.
Viruses
PCR not only allows a specific diagnosis in an individual patient but can also define the spectrum of disease caused by a particular virus and when applied to large numbers of clinical samples can help understand the epidemiology of neurological infections.
Several viruses can be looked for in the same CSF or other sample using the technique of multiplex PCR in which several pairs of primers specific for particular viral sequences are used. Where more than one virus is detected in a CSF sample, the significance of the virus detected has to be carefully evaluated, especially if such a dual viral infection is made more likely by immunosuppression as occurs during Human Immunodeficiency virus (HIV) infection
While evaluating PCR results the sensitivity and specificity of the particular assay are important factors to take into consideration ie. the possibility of false negative and false positive results. The timing of the CSF sample and the physical conditions such as specimen storage can be important
Using quantitative PCR real-time PCR allows the determination of the viral load in a patients' blood or CSF. Although this is not carried out routinely in most laboratories, it has been used in some cases to assess the severity of the viral disease burden and/or the prognosis, examples being Cytomegalovirus (CMV) and JC virus infections
Besides CSF Viral PCR can also be carried out on other tissues such as peripheral blood, brain, or other tissue biopsy specimens.
Herpes simplex virus
- The sensitivity of CSF PCR in HSE is 96% and the specificity is 99% . Hence CSF PCR can be recommended as a highly reliable method of diagnosing HSE without the need for brain biopsy (Level A).
- It is important to carry out CSF PCR for both HSV-1 and HSV-2 since HSV-2 is also associated with mild or atypical of cases of HSE, particularly in immunosuppressed patients such as those with HIV infection as well as neonatal HSE caused by HSV-2.
- Timing of PCR: The CSF PCR for HSE can be negative during the early stage of the infection. Re-examination of the same negative CSF a few days later may yield a positive PCR result . The CSF PCR may also be negative if the sample is taken too late during the infection since the yield of virus is highest during the first week of the infection following which it falls .The guidelines recommend the optimum timing of CSF PCR at 2–3 days to 10 days after symptom onset (Level C).
- If HSE is strongly suspected even if CSF PCR obtained within the first 72 h of the onset of symptoms is negative it should be repeated few days later to obtain a definitive diagnosis (Level B).
- Treatment with acyclovir does not reduce the chances of PCR detecting HSV during the first week of infection so such treatment should not influence the decision to carry out a PCR test (Level A). Once acyclovir has been started in a case of suspected HSE with a negative PCR, we recommend that it is continued for 14 days unless an alternative diagnosis has been established (Level C).
- Currently it is not recommended to repeat the CSF PCR in all patients after 14 days of acyclovir treatment. There is an on going US NIH anti-viral study group trial which also addresses this issue.
- Quantitative PCR: Routine Quantitattive PCR to assess the viral load has not been shown to be a useful prognostic marker in an individual patient with HSE and hence is not recommended.
Varicella-Zoster virus
- The sensitivity and specificity of VZV PCR in cases of VZV-associated neurological disease has been estimated at 80% and 98%, respectively.
- CSF PCR for VZV should be considered in all cases of encephalitis of unknown cause .
- PCR is also helpful in proving the etiology in a spectrum of neurological conditions caused by VZV without reactivation including VZV vasculopathy, zoster sine herpete, and myelopathy, meningoencephalitis, and polyneuritis cranialis. It is important to think of this possibility in differential diagnosis. In such cases in addition to CSF PCR for VZV DNA other tests for eg detection of anti-VZV IgG in the CSF should also be carried out.
- CSF anti-VZV IgG more sensitive than PCR in VZV vasculopathy
Cytomegalovirus
- CSF PCR has a very high sensitivity and specificity for detecting CMV infection and is recommended in patients with suspected CMV associated neurological disease.
- Quantitative CMV PCR may also be useful to correlate disease severity and monitor the efficacy of anti-viral therapy
Epstein–Barr virus
- EBV PCR on the CSF (Level C) is recommended in patients presenting with symptoms suggestive of the range of neurological conditions associated with EBV including encephalitis, aseptic neuritis, cerebellar ataxia, myelitis, and several peripheral nerve disorders including various types of acute radicultis, radiculoplexopathy, acute autonomic neuropathy, Guillain–Barre syndrome, and cranial neuropathies .
- The sensitivity of CSF EBV PCR in AIDS patients with a suspected CNS lymphoma is very high, at 97% -100%
- Quantitative PCR can also be used in such patients to predict the risk of developing non-Hodgkin CNS lymphoma and for monitoring the effects of chemotherapy
Enteroviruses
- Enteroviruses (EV) disease spectrum includes a non-specific febrile illness, aseptic meningitis, and encephalitis. A chronic meningoencephalitis may also occur in immunocompromised patients.
- CSF Enterovirus PCR is highly sensitive and specific in patients suspected of having an EV infection and recommended for both accurate diagnosis and improved patient management.
- Reverse transcription (RT)-PCR for EV provides a rapid and very accurate diagnosis of an infection in <24 h and much more quickly than is possible with standard viral culture (Class II).
- The CSF PCR for EV results should however be interpreted carefully in case of false negative results since some studies have shown that EV PCR of specimens from the respiratory and gastro-intestinal tracts yielded higher results than did CSF
- Obtaining a specific diagnosis is particularly important since patients may be potentially treated with the antiviral agent pleconaril.
JC Virus
- JC Virus (JCV) is a polyoma virus that is the causative agent of progressive multifocal leukoencephalopathy (PML), a demyelinating infection of the CNS that occurs mainly in immunocompromised individuals, primarily those with AIDS and recently also associated with natalizumab therapy in multiple sclerosis patients.
- The specificity of CSF PCR progressive multifocal leukoencephalopathy is excellent at 98.5–100% though the sensitivity is lower in the region of 50–82% (Class II).
- CSF PCR to detect JCV DNA is now the established and routine method of diagnosis in PML and has superseded brain biopsy
- In cases where the PCR is negative in a brain biopsy should be seriously considered to obtain a definitive diagnosis.
- Quantitative PCR is also helpful since higher CSF JC virus loads have been found to be associated with shorter survival times and viceversa. JC virus loads have also been used to monitor the effects of antiviral therapy in PML patients
Human immunodeficiency virus
- Diagnosis will already have been made on the blood
- Quantitative PCR to measure the CSF viral load has been a valuable tool in assessing neurological involvement in HIV infection such as HIV-associated dementia and encephalitis and also to monitor therapy as the CSF viral load decreases markedly following highly active antiretroviral therapy (HAART).
Human T-cell lymphotropic virus-1
- Human T-cell lymphotropic virus-1 (HTLV-1) is strongly associated with tropical spastic paraparesis and HTLV-1-associated myelopathy
- Use of PCR is recommended (Level C) in the diagnosis of tropical spastic paraparesis and HTLV-1-associated myelopathy.
- The sensitivity and specificity of CSF PCR for tropical spastic paraparesis and HTLV-1-associated myelopathy has been reported as 75% and 98.5%, respectively. Combination of CSF PCR and anti-HTLV-1 antibody index useful in diagnosis (Class III Level C)
Bacteria
The PCR results are usually available well within 24–36 h for common bacterial infections and utilize low volume of CSF (≥1 ml) for analysis.
PCR methods may employ one of the several available techniques:
- Nested or semi-nested PCR with hybridization and sequencing, or use of universal primers and restriction endonuclease enzyme digestion. Nested approach is considered better in detecting meningeal infections with Borreila, Listeria, or Mycoplasma because of the low numbers of bacterial DNA and relatively few copies of 16S rRNA gene in the CSF
- Probe-based real-time PCR is often preferred when using a multiplex PCR for simultaneous detection of different specific target sequences. Real-time quantitative multiplex PCR is considered a highly sensitive technique for fast identification of a causative pathogen of bacterial meningitis and can detect as few as two copies of Neisseria meningitidis, Streptococcus pneumoniae, and Escherichia coli, 16 copies ofListeria monocytogenes, and 28 copies of group B streptococcus whereas the sensitivity for broad-range 16S rRNA-based PCR was about 10–200 organisms per ml of CSF
- Broad-range bacterial PCR is based on use of primers that recognize conserved regions of the genes encoding for eubacterial 16S ribosomal RNA (rRNA). The broad-range bacterial PCR combined with sequencing may be of particular advantage in rapid diagnosis and identification of the etiologic agent in community acquired bacterial meningitis .
Acute meningitis
- Currently available PCR methods detect Hemophilus influenzae, N. meningitidis, S. pneumoniae, L. monocytogenes in CSF and have a sensitivity of 87–100% and specificity of 98–100%
- Quantitative multiplex RT-PCR is commonly preferred for the detection of common pathogens of acute bacterial meningitis.
- PCR-based detection of bacterial pathogens is also considered more sensitive than culture in patients with ventricular catheters and suspicion of nosocomial meningitis or conventional cultures withprior antibiotic treatment
- The positive predictive value of broad-range PCR is 98%, and the negative predictive value is 100%; ie a negative bacterial PCR assay virtually excludes the diagnosis of acute bacterial meningitis.
- It is recommended that in house nucleic acid amplification methods for diagnosis of bacterial infections in CSF are deemed unreliable and should not be used in clinical practice (Class IV Grade C) owing to high inter-assay variability and low specificity
- The advantage of uniplex vs multiplex quantitative RT-PCR is currently unclear (Class IV Grade C).
- PCR-based diagnostic tools should be used only as an adjunct rather than a substitute for current methods of bacteriological diagnosis by conventional staining and culture.
- In Future: it might be possible to rapidly diagnose bacterial infections by Gram stain-specific probe-based real-time PCR using 16S rRNA which will enable simultaneous detection and discrimination of clinically relevant Gram-positive and Gram-negative bacteria directly from blood samples
Chronic meningitis
- Tuberculous Meningitis:
- Quantitative RT-PCR has been shown to substantially increase diagnostic yield in TBM.
- A wide range of sensitivities have been reported for PCR-based tests forMycobacterium tuberculosis in CSF samples .
- Using CSF filtrate than CSF sediment has been reported to give higher yield of positive result qualitative RT-PCR.
- Since PCR-based methods are also prone to cross-contamination like conventional cultures, and the diagnostic specificity of PCR-based diagnosis of TBM may be compromised in endemic areas .
- A negative CSF PCR result does not exclude the diagnosis of neurotuberculosis when CSF and imaging data suggests otherwise and one must be aware of false positives as well.
- In acute bacterial meningitis , CSF rapidly becomes sterile after antibiotic therapy and bacterial DNA may not be detectable beyond 8 h of treatment . However Mycobacterial DNA may persist for up to a month in CSF after starting therapy .Hence even if initial PCR result is negative, repeating quantitative RT-PCR test in successive CSF samples for M. tuberculosis may aid to diagnosis even if initial PCR result is negative
- The current consensus is that repeating CSF PCR within first 3 weeks may aid diagnosis in tuberculous meningitis if the initial result is negative (Class IV, Grade C).
- Lyme neuroborreliosis:
- In Lyme neuroborreliosis CSF PCR for Borrelia is probably useful as a diagnostic test only in very early stages and is not recommended as a diagnostic test for chronic Lyme disease or measure treatment response as a follow-up.
- However in patients with atypical forms of erythema migrans PCR on skin biopsies may become useful in the diagnosis of early Lyme borreliosis.
- CSF- PCR is not presently a validated diagnostic test for Lyme neuroborreliosis (Class IV, Grade C).
- Direct microscopy and culture remain the gold standard of microbiological diagnosis of bacterial infections of central nervous system where feasible and current range of diagnostic bacterial PCR tests do not replace them (Class II Grade A).
- Commercially available and standardized quantitative RT-PCR is recommended for routine use in CSF samples (Class II, Grade A) of patients with suspected bacterial meningitis.
Parasites
- The diagnosis of a parasitic infection of the CNS rests upon clinical signs and symptoms, clinical history, travel history including geographic exposure and, finally, on laboratory techniques.
- In resource poor areas light microscopy remains the diagnostic mainstay.
- Indirect methods like serology do not distinguish between past, latent, reactivated, or acute infection and is not useful in assessing response to therapy or prognosis.
- In immuno-compromised patients, indirect diagnostic methods (serology for antibody detection, etc.) have a very low sensitivity.
- Highly specific tests to detect antigen, for example, rapid antigen detection system (RDTS) luciferase immune precipitation system (LIPS), molecular-based approaches, in particular PCR , loop-mediated isothermal amplification (LAMP) , real-time (RT) PCR , and luminex technology have shown a high potential for use in diagnosing parasitic infestations with increased sensitivity and specificity .
Molecular-based diagnostic tests in protozoal infections of the CNS
Table below lists the protozoa that have the capacity to invade the CNS, causing neurological disease and details the molecular-based techniques used in their detection. Except cerebral toxoplasmosis the other molecular-based assays are still experimental diagnostic techniques and have not yet replaced serology or direct proof by light microscopy.
|
Protozoal pathogen |
CNS manifestation |
Molecular-based diagnostic technique |
|
Free living amebae |
Granulomatous amebic encephalitis |
PCR |
|
Entamoeba histolytica |
Brain abscess |
Real-time PCR |
|
Babesia microti |
Anemia, hypoxic encephalopathy |
PCR |
|
Plasmodium falciparum |
Cerebral malaria, multi-organ malaria |
Real-time PCR |
|
Plasmodium knowlesi |
Usually severe anemia |
Nested PCR |
|
Toxoplasma gondii |
Cerebral toxoplasmosis (granulomata, acute encephalitis; very rare in immuno-competent, usually in immuno-compromised patients) |
Quantitative polymerase chain reaction |
|
Trypanosoma cruzi |
Acute meningoencephalitis, myocarditis, in chronic Chagas disease: cardio embolic stroke |
PCR |
|
Trypanosoma brucei gambiense and Trypanosoma bruceirhodesiense |
Chronic (T. b. gambiense) or sub-acute (T. b. rhodesiense) meningoencephalitis |
PCR |
Molecular-based diagnostic tests in helminthic infestations of the central nervous system
- Conventional PCR has been supplemented by nested and multiplex PCR as well as real-time PCR for the detection of several parasitic infestations and infections, respectively.
- More advanced techniques as loop-mediated isothermal amplification (LAMP) and luminex-based assays have also been proposed as possible diagnostic techniques in parasitic diseases of the nervous system.
- Direct visualization or detection of the helminths, either the adult worm, the larval stage, or the eggs, be it in body fluids or biopsied material, still represents the golden standard of diagnosis
|
Helminth |
CNS manifestation |
Molecular-based diagnostic technique |
|
Angiostrongylus cantonensis |
Eosinophilic meningitis |
Multiplex PCR |
|
Echinococcus granulosus |
Cystic echinococcosis (space-occupying intracranial cyst) |
Direct-PCR |
|
Filarial species |
Lymphatic filariosis, rarely: neurofilariasis (cerebral larva migrans) |
Real-time PCR |
|
Paragonimus westermani |
Space-occupying intracranial cyst |
Multiplex PCR |
|
Schistosoma spp. |
Space-occupying granuloma intracerebral and spinal space- occupying granuloma |
PCR |
|
Strongyloides stercoralis |
Strongyloides stercoralis hyperinfection syndrome (in the immune-compromised) with fulminant meningitis and sepsis syndrome (accompanying gram negatives) |
PCR |
|
Taenia solium – larval stage:Cysticercus cellulosae |
Neurocysti-cercosis (Space-occupying, cystic intracranial lesions, epilepsy) |
PCR |
|
Toxocara canis(cati) |
Larva migrans visceralis (cerebralis, intracranial granuloma, vasculitis) |
PCR |
Fungi
The gold standard of diagnosis is positive cultures together with microscopy, antigen/antibody testing in serum, and CSF. However the slow growth of fungi in culture, cross-reactivity in case of antigen detection, and dependence on the demonstration of an antibody response or even by the failure to mount an adequate immune response are drawbacks. There is not enough data to recommend the routine use of PCR in fungal infections.
Histoplasmosis (Histoplasma capsulatum) :
- Most common endemic mycosis in Europe
- Chronic meningitis is the most frequent CNS manifestation of histoplamosis
- Cerebral or spinal masses and encephalitis are less common.
- Fungal culture is the gold standard diagnostic test in non-CNS manifestations, but it may take up to 6 weeks
- CSF cultures usually do not yield growth.
- Histoplasma capsulatum antigen and antibodies can be determined by different methods in CSF and are used to establish diagnosis. However, cross-reactivity with other dimorphic fungi and Cryptococcus species is seen in upto 50% of cases.
- There is no commercial kit available for routine use for PCR in CNS histoplasmosis
Coccidioidomycosis (Coccidioides immitis)
- Chronic basal meningitis is the most common coccidioidal CNS manifestation.
- Occasionally, CNS coccidioidomycosis presents as meningoencephalitis or intracranial mass lesion.
- Coccidioides sp. grows in culture within 2–5 days
- Coccidioides sp. is isolated from CSF only in a third of patients with CNS manifestations
- CSF antibodies can be detected in up to 70% of patients with meningitis during the initial analysis, and in the majority of cases later.
- Currently, there are no standardized CSF antigen detection methods
- No PCR is available for commercial use.
Blastomycosis (Blastomyces dermatitidis)
- CNS manifestations of blastomycosis are rare, mostly cranial/spinal epidural abscess, meningitis, and brain abscess in AIDS patients.
- Definitive diagnosis of blastomycosis requires growth of the organisms in culture.
- CSF culture is rarely positive, and only stereotactic brain biopsy may be diagnostic.
- cytological examination of CSF may sometimes reveal Yeast forms.
- CNS blastomycosis may be diagnosed by presence of antibodies in serum and CSF, cross-reactivity with other fungi is possible.
- Chemiluminescent DNA probes have been developed but not standardised or commercially available.
Cryptococcosis (Cryptococcus neoformans/gattii)
- Chronic basal meningitis is the most frequent CNS manifestation of cryptococcal disease causing subacute dementia or visual symptoms.
- Culture alone is generally not the method of choice.
- The diagnostic mainstay is antigen detection with >90% sensitivity and specificity. This test in CSF can be positive early in infection.
- Microscopic examination of CSF using India ink stain is diagnostic in up to 80% of AIDS patients and about 50% in non-immunocompromized.
- The usage of CSF PCR for the diagnosis of suspected CNS cryptococcosis is likely to be of value
Candidiasis (Candida albicans and other C. species)
- Candida species are the fourth leading cause of fungal bloodstream infections and associated with a mortality rate of up to 50%.
- CNS Candidiasis can develop in the setting of disseminated candidiasis with microabscesses of the brain parenchyma or as candida meningitis in association with a foreign body (e.g. catheter, ventricular shunt) or other CNS invasive procedures (e.g. surgery).
- Blood cultures are considered the gold standard for routine diagnosis of invasive candidiasis but are time-consuming.
- CSF, stains and routine cultures have a low yield in identifying the pathogen in chronic Candida meningitis.
- Serological diagnosis of CNS candida infection, either by antigen detection (mannan) or by antibody determination, has not been validated.
- PCR protocols for detection of candidal infection have been described and require probes for different subspecies. PCR may also assess mutations associated with resistance to antifungal medication.
Aspergillosis (Aspergillus fumigates and other species)
- There are numerous species of aspergillosis recognized, but most cases of CNS infection are attributed to A. fumigates, A. flavus,A. terreus, and A. versicolor. Major CNS manifestations include hemorrhagic infarction, abscess, and meningitis; less frequent are mycotic cerebral aneurysm and granuloma.
- Culture is insensitive and diagnosis requires non-culture-based methods.
- Two antigen assays, the [1,3]-beta-D-Glucan assay (sensitivity 87%), a relatively non-specific assay and the galactomannan assay (>95% sensitivity and specificity), an Aspergillus-specific antigen, are commercially available and used in clinical routine for blood specimen and sometimes in CSF samples.
- A combination of the galactomannan (antigen) assay and PCR may improve diagnosis.
- The sensitivity of PCR testing for Aspergillus is limited during antifungal treatment.
- The usage of CSF PCR for the diagnosis of suspected CNS aspergillosis is likely to be of value
Mucormycosis/zygomycosis (Mucor, Rhizpous, Rhizomucor, Absidia, Cunninghamella, Apophysomyces, Saksenaea)
- Mucormycosis is an acute and aggressive fungal infection, which can develop as isolated cerebral mucormycosis (16%), extension to the brain from rhinocerebral mucormycosis (69%) or via the hematogenous route (15%).
- Diagnostic techniques include histology, culture, and PCR. The diagnosis is mostly made by a combination of histology and culture.
Conclusion
- The main use of PCR technology is to the diagnosis of infections caused by viruses followed by bacterial infections of the CNS with the notable exception of tuberculous meningitis.
- The efficacy of PCR for the diagnosis of both protozoal infections and helminthic infestations has also been established but far from becoming routine in resource-poor countries where such infections are prevalent.
- There are class IV evidence studies reporting the feasibility of CSF PCR for evaluating CNS manifestations by Histoplasma, Coccidioides, and Candida, and of tissue for CNS mucormycosis. There is not enough evidence at present to recommend the use of PCR as a routine diagnostic tool in these cases.
Original citation:
Steiner, I., Schmutzhard, E., Sellner, J., Chaudhuri, A. and Kennedy, P. G. E. (2012), EFNS-ENS guidelines for the use of PCR technology for the diagnosis of infections of the nervous system. European Journal of Neurology, 19: 1278–1291. doi: 10.1111/j.1468-1331.2012.03808.x [ Abstract]
Other resources:
Read More
- Details
- ICNA
- News
- Hits: 736