- Details
- ICNA
- News
- Hits: 684
 A new journal of Epilepsy the North African and Middle East Journal of Epilepsy has started publication. The journal will be the mouthpiece of the Leagues and Associations of the East Mediterranean Region of the International Bureau of Epilepsy and The International League Against Epilepsy. From Mauritania in the West to Pakistan in the East, this covers 20 countries in Northern Africa and the Middle East.
A new journal of Epilepsy the North African and Middle East Journal of Epilepsy has started publication. The journal will be the mouthpiece of the Leagues and Associations of the East Mediterranean Region of the International Bureau of Epilepsy and The International League Against Epilepsy. From Mauritania in the West to Pakistan in the East, this covers 20 countries in Northern Africa and the Middle East.
National Leagues or Associations represented by the journal include the Algerian League Against Epilepsy, Egyptian Society Against Epilepsy, Jordanian League Against Epilepsy, Kuwait League Against Epilepsy, Lebanese League Against Epilepsy, Qatar League Against Epilepsy, Moroccan Association Against Epilepsy, Moroccan League Against Epilepsy, Tunisian Association Against Epilepsy, Saudi Chapter of Epilepsy, Sudanese League Against Epilepsy, and Syrian League Against Epilepsy.
Najib Kissani from University Cadi Ayyad of Marrakech, is the editor-in-chief of this new journal ,with articles published in French or English. Currently six issues and 2 supplements per year are proposed. Dr. Kissani says that this new journal addresses several unmet regional needs. There is a wide disparity in epilepsy care among the countries of North Africa and the Middle East.
Egypt has 400 neurologists, whereas Mauritania has 3. Even affluent countries, such as Algeria, the Gulf countries, Lebanon, and Tunisia, have only 1 neurologist for 35,000 residents, while poorer countries, such as Mauritania, Somalia, and Yemen, have less than 1 neurologist per 2 million persons. There are several unmet needs when it comes to care of neurological disorders in these regions which this new journal hopes to cover. Epilepsy still carries a lot of stigma in these countries and for want of better resources, several preventable causes of epilepsy still remains untackled.
The three current regional neuroscience journals, Neurosciences (Saudi Arabia), Current Alzheimer Research (United Arab Emirates), and CNS & Neurological Disorders Drug Targets (United Arab Emirates), are not particularly focused on epilepsy. The North African and Middle East Journal of Epilepsy will provide a platform for regional physicians to disseminate their clinical experience and research , though submissions are invited from physicians all over the world.
Besides scientific articles, other contributions including stories experienced by patients with epilepsy, physicians or other professionals involved in epilepsy" and news from regional societies, leagues, and associations against epilepsy are also invited. Articles are currently invited in either French or English. The journal is not currently indexed in Medline.
The inaugural issue included an editorial on the state of epilepsy in the North African and Middle East region in light of the Arab Spring, a case report of polymicrogyria, a history of the Tunisian Association Against Epilepsy, and articles on epilepsy in Mali and Gambia. The journal is sponsored by the University Cadi Ayyad of Marrakech, Moroccan Association Against Epilepsy, Moroccan League Against Epilepsy, and the Watania Press. The staff includes 12 associate editors, 2 editorial assistants, and 45 reviewers from multiple countries. There are both print and online editions and the subscription is fee based.
Download the January 2012 issue here
Read More
- Details
- Biju Hameed
- News
- Hits: 648
 A new generation of implantable devices called the "Closed-loop" devices are designed to monitor the seizure focus, detect patterns of electrical activity suggestive of seizures, and automatically respond in the form of electrical stimulation , cooling or focused drug delivery inorder to interrupt and stop the seizure activity.
A new generation of implantable devices called the "Closed-loop" devices are designed to monitor the seizure focus, detect patterns of electrical activity suggestive of seizures, and automatically respond in the form of electrical stimulation , cooling or focused drug delivery inorder to interrupt and stop the seizure activity.
The Vagal Nerve Stimulator (VNS) is on the other hand an open-loop device that stimulates the vagus nerve and deliver consistent mild electrical pulses to the brain. Unlike closed loop devices it does not stimulate in response to a seizure. Cyberonics' Vagus Nerve Stimulator (VNS) has had FDA approval for treatment of epilepsy since 1997.
NeuroPace's Responsive Neurostimulation System (RNS), is an example for a closed-loop device that has now reached human-trials. The RNS electrical-stimulation implant has two leads, each containing four electrodes, which are placed in the brain at the seizure focus.
The RNS detects electrical activity that denotes the start of a seizure and delivers direct electrical stimulation to interrupt the activity and normalize the area. The device is surgically positioned in the skull and can be accessed via outpatient surgery in order to change batteries etc. The device is also capable of recording continuous information on the electrical activity of the brain and has a laptop-based wand interface for remote patient monitoring.
However the RNS trial where the device was tested in conjunction with medications, have only given mixed results with the seizure frequency reduced by about half in only approximately half of the patients studied.
There is also on going research to turn the VNS into a closed-loop device by developing a non implanted add-on system to detect early seizure activity and automatically fire the VNS in response. The therapy magnet wristband which comes with the VNS could be used by the wearer to stimulate the device if they feel a seizure coming on . However not everyone who suffer from seizures is able to physically stimulate the device even if they feel an aura.
The Center for Integration of Medicine and Innovative Technology (CIMIT ) at Boston is working on a way of automating this process, activating the VNS once the start of a seizure by detecting changes in EEG or ECG.
Focal cooling: The closed loop device can also after detecting the onset of a seizure by sensing a rise in brain temperature at the seizure focus, can rapidly cool the involved region in order to stop the seizures. This can be more advantageous since the rise in temperature may happen slightly before the onset of abnormal electrical activity.
 ,Ivan Osorio Professor Ivan Osorio University of Kansas Medical Center is currently collaborating with an international research partnership to design a prototype implant with funding from the U.S. Department of Energy. Research on similar projects is also happening at Yale and Minnesota universities.
,Ivan Osorio Professor Ivan Osorio University of Kansas Medical Center is currently collaborating with an international research partnership to design a prototype implant with funding from the U.S. Department of Energy. Research on similar projects is also happening at Yale and Minnesota universities.
Targeted drug delivery: The closed-loop devices could also use convection-enhanced drug delivery (CED) .Here the antiepileptic drugs are delivered directly to specific brain areas through an implanted catheter. The device could also be used to deliver a regular infusion of the drug which migh be more helpful than triggering in response to a seizure.
Michael Rogawski, chair of neurology at the University of California, Davis, whose lab is working with British Columbia–based biopharmaceutical company MedGenesis Therapeutix to develop an implantable CED device for epilepsy.
Devices which are used for Deep Brain Stimulation are also open-loop devices where the implanted devices is used to consistently stimulate the anterior nucleus of the thalamus in order to control the cortical electrical excitability. DBS might be helpful in patients where the seizure activity originates from a widespread area
However there are several risks associated with these implantable devices. The main concern besides those inherent with the surgery, are regarding false positives, where beneficial electrical activity is falsely interrupted upon. Clearly the challenge is to devise a gadget which can predict the seizures accurately, early and act fast enough to stop them.
Source: Scientific American
Read More
- Details
- Biju Hameed
- News
- Hits: 605
A study from Boston Children's Hospital published in BMC Medicine this week suggest a EEG coherence-based phenotype for childhood autism.
EEG coherence is a measure of the degree of association or coupling of frequency spectra between two EEG signals when compared over time.
The EEG coherence was studied by quantifying the degree to which any two EEG signals from multiple electrodes at different time points were synchronised.High coherence values for eg where two or more waves rise and fall together over time, are taken as a measure of strong connectivity between the brain regions that produce the compared EEG signals.
The investigators compared raw EEG data from 430 children with autism and 554 control subjects, ages 2 to 12, and found that those with autism had consistent EEG patterns indicating altered connectivity between brain regions.
Using computational techniques, the researchers generated coherence readings for more than 4,000 unique combinations of electrode signals, and looked for the ones that seemed to vary the most from child to child. From these, they identified 33 coherence "factors" that consistently distinguished the children with autism from the controls, across all age groups (2 to 4, 4 to 6, and 6 to 12 years).
The autistic children generally showing reduced connectivity compared to controls. The left hemisphere language areas in particular showed reduced connectivity in autistic children, consistent with neuroimaging findings suggesting altered connectivity in the arcuate fasciculus
There are several interesting aspects to the study methodology in that the researchers had included a mid-range cross section of childhood autism and pervasive developmental disorder while excluding the high functioning autism or Asperger's syndrome where the children are typically cooperative. The group at Boston children's also had at their disposal a large database of EEG data on typical neurologically normal children.
In addition they had also studied all the available scalp channels and spectral bands and using a technique called Principal Components Analysis the extensive spectral coherence data sets were reduced to a much small number of factors and these variables were studied among the cases and controls.
There are plans to repeat this study in children with Asperger's syndrome and also in autism associated with tuberous sclerosis, fragile x syndrome and extreme prematurity and see if its EEG patterns are similar to or different from that of classic autism.
The findings from this study are interesting since it explores potential diagnostic tests for autism particularly at younger ages when behavior-based measures are unreliable.
The full text of this study is available via open access at http://www.biomedcentral.com/1741-7015/10/64
Source: Children's Hospital Boston
Read More
- Details
- ICNA
- News
- Hits: 799
MINNEAPOLIS – The American Academy of Neurology has issued an updated guideline outlining the best treatments for infantile spasms. The guideline, which was co-developed with the Child Neurology Society, is published in the June 12, 2012, print issue of Neurology®, the medical journal of the American Academy of Neurology.
The guideline found that the hormone therapy adrenocorticotropic hormone, also known as ACTH, may be effective for treatment of infantile spasms. The seizure drug [[vigabatrin]] may also be considered for treatment, although evidence suggests ACTH may be more effective than vigabatrin. For children with seizures caused by tuberous sclerosis complex, however, vigabatrin may be more effective.
The guideline, which is based on a review of all available evidence on treatment for infantile spasms and is an update of a guideline published in 2004, also found that low-dose ACTH is probably as effective as high-dose ACTH and it may lower the risk of side effects.
There is not enough evidence to know whether other treatments, alone or combined, are effective in treating infantile spasms, according to the guideline.
The guideline recommends that early diagnosis and early treatment may lead to better long-term outcomes for children's development and learning skills.
Guideline
Update: Medical Treatment of Infantile Spasms
Read More
- Details
- Biju Hameed
- News
- Hits: 608
An important work published in the May 24 issue of Neuron by, researchers from Dana-Farber Cancer Institute and Harvard Medical School helps toward the understanding of the molecular mechanisms of mitochondrial and metabolic control of seizures and helps to uncover how the ketogenic diet works in epilepsy.
The efficacy of the ketogenic diet in children with epilepsy has pointed to a role for metabolism as a component of the pathogenesis of seizures. However the molecular mechanisms behind the dependence of neuronal activity on metabolic substrates has not been fully understood2. In parallel to its role in apoptosis, the Bcl-2-associated death promoter (BAD) protein , a pro-apoptotic member of the Bcl-2 gene family, has been shown to have an important role in glucose metabolism and utilization.
In this respect the phosphorylation of BAD does not only prevent initiation of cell death, but it is also required for efficient mitochondrial utilization of glucose in liver. Dephosphorylated BAD forms a heterodimer with Bcl-2 and Bcl-xL, inactivating them and thus allowing Bax/Bak-triggered apoptosis.
Giménez-Cassina et al. (2012) investigating the potential role of BAD in seizures, found the existence of a phosphodependent regulatory switch in BAD that reduces neuronal excitability upon kainic acid-induced seizures. Similar to what occurs in the liver they showed that cortical neurons and astrocytes from Bad−/− mice display lower glucose utilization for mitochondrial respiration.
This showed an interesting connection between BAD deficient mice or mice with nonphosphorylable BAD and ketogenic diet which reduces seizures in terms of glucose utilization by mitochondria.
The authors therefore investigated whether BAD might also influence seizure sensitivity in vivo. They found that BAD deficient as well as BadS155A(mice bearing a phosphodeficient knockin allele of Bad at serine 155) mice were significantly protected from the proconvulsant drug kainic acid. Moreover, seizure resistance was specific for BAD and independent from its proapoptotic function, pointing therefore to its role in metabolism.
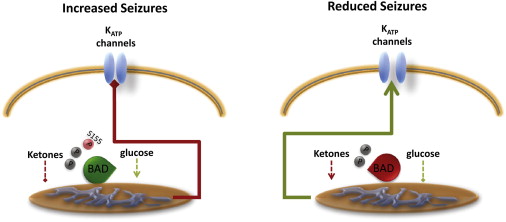 BAD Phosphorylation Controls Mitochondrial Fuel Utilization to Switch Neuronal ExcitabilityThe proapoptotic BH3-only protein BAD can be viewed as a relay: its phosphorylation on Ser 155 switches the preferred source of reducing equivalents for mitochondria from glucose to ketones. Plasma membrane KATP channels are closed in neurons with “glycolytic” mitochondria, as opposed to the ones where mitochondria use ketone bodies and that are less susceptible to seizures. (source: Neuron Volume 74, Issue 4, 24 May 2012, Pages 600–602)The Ketogenic diet has been shown to increases the activity of KATP channels. KATP channels are activated following decreased intracellular ATP, in a negative feedback loop that is believed to help neurons to overcome excitotoxicity during seizure. Giménez-Cassina et al. also found that the dentate granule neurons (DGNs) of hippocampal slices from BAD deficient (Bad−/− ) mice showed increased single KATP channels, while ablation of KATP channels expression in BAD deficient (Bad−/− ) mice diminished their resistance to seizures .
BAD Phosphorylation Controls Mitochondrial Fuel Utilization to Switch Neuronal ExcitabilityThe proapoptotic BH3-only protein BAD can be viewed as a relay: its phosphorylation on Ser 155 switches the preferred source of reducing equivalents for mitochondria from glucose to ketones. Plasma membrane KATP channels are closed in neurons with “glycolytic” mitochondria, as opposed to the ones where mitochondria use ketone bodies and that are less susceptible to seizures. (source: Neuron Volume 74, Issue 4, 24 May 2012, Pages 600–602)The Ketogenic diet has been shown to increases the activity of KATP channels. KATP channels are activated following decreased intracellular ATP, in a negative feedback loop that is believed to help neurons to overcome excitotoxicity during seizure. Giménez-Cassina et al. also found that the dentate granule neurons (DGNs) of hippocampal slices from BAD deficient (Bad−/− ) mice showed increased single KATP channels, while ablation of KATP channels expression in BAD deficient (Bad−/− ) mice diminished their resistance to seizures .
The study thus linked BAD phosphorylation and KATP channels activity to the attenuation of seizures, a hitherto unknown signalling pathway The study, funded by Harvard Catalyst, Citizens United for Research in Epilepsy, and the NIH has contributed significantly to the understanding of the molecular mechanisms of mitochondrial and metabolic control of seizures with potential implications for future therapeutic strategies.
Citations
1. Elena Ziviani, Luca Scorrano. Neuron, Volume 74, Issue 4, 24 May 2012, Pages 600-602
2. Giménez-Cassina et al. Neuron, 74 (2012), pp. 719–730
Read More