- Lectures / Webinars
- Monoclonal antibodies in Multiple Sclerosis
Monoclonal antibodies in Multiple Sclerosis
Hot
 |
The following table essentially highlights the relative efficacies of MS therapies. And you can see that the top four treatments, which are all monoclonal antibodies, have a higher efficacy. What you have to appreciate is most of these treatments have been tested against active comparators; therefore, the relative efficacy is much higher than those treatments that have been compared to placebo. The efficacy differential is present on relapses—this is the annualised reduction in relapse rate.
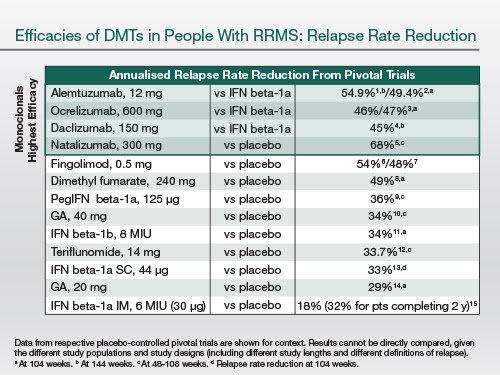 |
And also [there is an efficacy differential] on disability progression. And the natural history studies, now that we're seeing [these] in people on more effective disease-modifying treatments, confirm that these results are extending out many years into clinical practice.
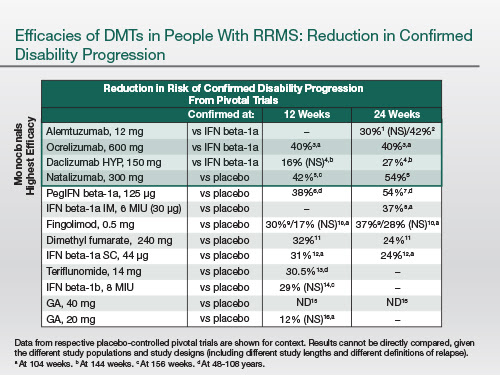 |
So the big question is why do monoclonals actually work better than other licensed disease-modifying treatments? And this is a subject for debate. But I do think an antibody that targets a specific protein or target is much more likely to give a therapeutic benefit, as it has very few off-target side effects. This is one of the problems with small molecules, and this actually limits optimisation of doses with small molecules.
And because you can target key pathogenic processes in the causal pathway of MS, you're likely to hit pivotal points and therefore, switch off the disease processes.
So these monoclonal antibodies provide us with a lot of insights, so let's just go through them.
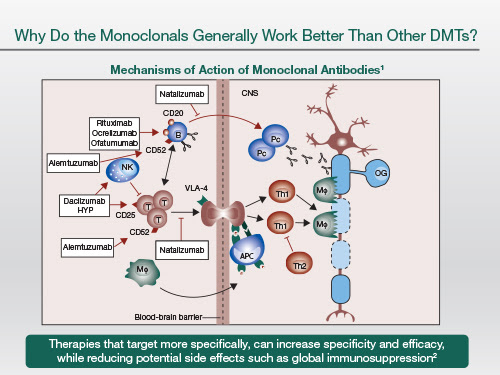 |
| So natalizumab essentially blocks lymphocyte trafficking into the central nervous system; it targets both T and B lymphocytes. So that's clearly a critical process in the pathogenesis of MS |
There's potentially another mode of action yet to be identified fully, [which] is that the plasma cell that resides within the central nervous system and produces antibodies lives inside a plasma cell niche, and that niche is partly dependent on [the] VCAM-1/α4 β1-integrin interaction. And if you block that interaction within the niche, you may actually deprive the plasma cell of survival signals. And so natalizumab may be working in the long-term against intrathecal plasma cells, which are implicated in the pathogenesis of MS, particularly progressive MS [Harrer A et al. Mult Scler. 2013;19:1209-1212; Bonnan M. Mult Scler Int. 2015;2015:296184].
So, because it blocks trafficking not only of autoreactive but of normal lymphocytes, it reduces the immune surveillance within the central nervous system. And the main side effect to emerge is opportunistic infection—progressive multifocal leukoencephalopathy that [sometimes] occurs in people who are infected with the JC, or John Cunningham, virus.
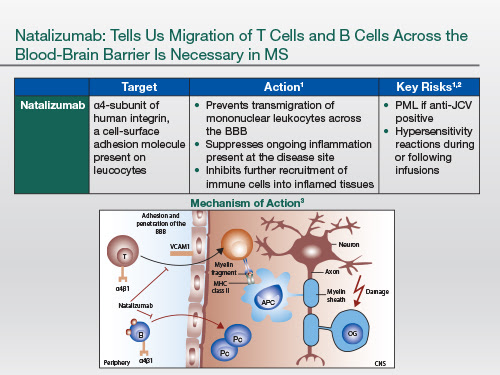 |
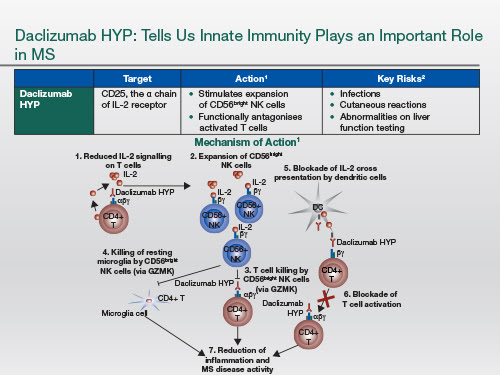
Daclizumab HYP binds to the high-affinity IL-2 receptor, which is mainly expressed on activated T cells and T regulatory cells. And by blocking this receptor, it actually allows IL-2 to work on the intermediate-affinity receptor, that's particularly expressed on the NK CD56bright cell, which is part of the innate immune system. This allows this particular cell type to expand, and this has immunoregulatory activities; in other words, it goes back and kills autoreactive T cells, both CD4+ and CD8+.
This is a very interesting drug because it's not one that targets specifically the B cell, but when we look at the spinal fluid of people with MS who are on daclizumab HYP, the number of B cells that are present has dropped [to normal levels]. So it must have some downstream or knock-out effects on the B cell compartment.
The main side effect would be infections. But it appears once the immunity is triggered, the infections are manageable.
We have seen a few cases of autoimmune hepatitis. These are quite rare, but they still will need to be monitored for.
 |
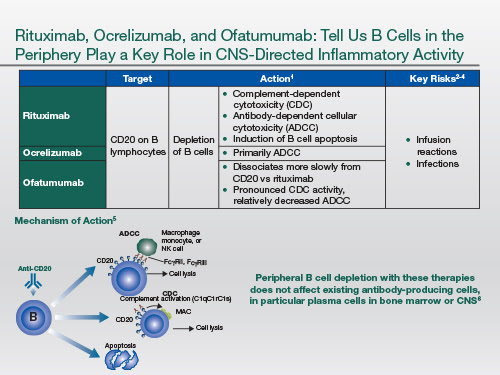
So coming to the anti-CD20 therapies, they work as depleting antibodies by antibody-dependent cellular cytotoxicity. And some of them are also complement-binding. And by inducing conformational changes in the anti-CD20 molecule, they also can trigger apoptosis of the cell.
Now, the interesting thing about anti-CD20 therapies is they work mainly by depleting B cells in the periphery. We know from CSF studies that the numbers of B cells in the spinal fluid go down, but plasma cell blasts, which are a precursor of the plasma cell pool, are not affected by anti-CD20, because they don't express CD20 on their surface. And so there is a question mark about whether or not the plasma cell pool also needs to be targeted in MS, because there is some evidence that the follicle-like structures that occur in the meninges and the cortex of the brain of people with MS are linked to progressive MS and cortical lesions.
Ocrelizumab: its main side effect from the trial development programme has been infusion reactions.
 |
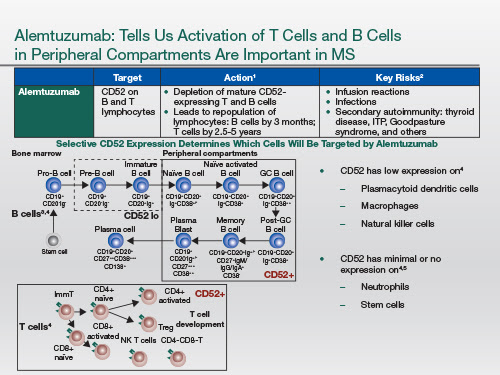
Alemtuzumab targets the cell surface receptor called CD52 that's expressed on most leukocytes, but the density of the receptor varies depending on cell type; and so most of these T and B lymphocytes that are depleted by CD52 over the long term are the ones with the highest density. So when you give this drug by intravenous infusion, you get a marked leucocyte depletion. But the cells with low levels of expression repopulate very quickly, and these include the mononuclear cells as well as the innate immune cells. The B cell lymphocyte population repopulates very, very quickly; whereas the T cell component, both the CD4+ and CD8+, take much longer to repopulate.
We think the repopulation kinetics, the B cells coming back long before the T cells, may be responsible for the secondary autoimmunity that occurs as a result of being treated with this drug. Autoimmune thyroid disease occurs in about 30% of people. But more importantly are immune thrombocytopenic purpura, ITP, and Goodpasture syndrome where you make antibodies to the glomerular basement membrane. And these can be life-threatening complications. As a result of this, when you use alemtuzumab, patients have to have monthly blood tests and urine tests to monitor for these complications, because if they're detected early they're potentially treatable.
 |
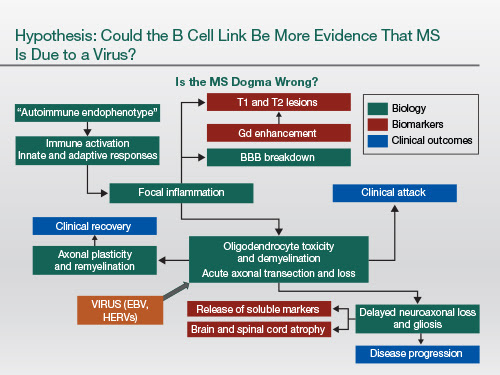
Now I'd like to make the argument that all four of these monoclonal antibodies appear to have some effect on B cells. And that's important because these treatments have told us unequivocally that the B cell must be a pivotal cell in the pathogenesis of MS.
And you've got to ask the question: Is there something else apart from the autoimmune hypothesis that explains this? This particular cartoon that I've drawn up to explain the current MS dogma implies that everything is driven by the so-called T lymphocyte, particularly the CD4+ infiltrate.
We must remember, though, that this could all be secondary to an infective cause. Say if, for example, we had a virus that infected oligodendrocytes directly or infected the axon directly, maybe the virus is triggering the immune response to itself. And all this T cell biology could be secondary. And the viruses that have got the most data behind them supporting a role in the causal pathway are Epstein-Barr virus and human endogenous retroviruses (HERVs).
What's very interesting is EBV infection actually brings the HERVs out of the genome and makes them multiply. So we can link the biology of these two viruses together, and we can also put into the hypothesis that B cell depletion targets Epstein-Barr virus because that's the cell that EBV resides in, long term. And therapy that upregulates the innate immunity, for example, NK cell function, may also be activating antiviral pathways that target the viruses.
 |
So to conclude monoclonal antibodies have ushered in a really exciting new era for the study of MS and [have] taught us a lot about the pathogenesis of MS—which are key pathological processes and how to target them more precisely. As a result of this, we've got much higher efficacy; and all the monoclonals now that are emerging have well-defined safety profiles. And we have paradigms for monitoring and de-risking these treatments. Clearly, there may be some safety trade-offs and this will impact on which ones we choose, but we'll have to individualise treatment decisions.
 |
Author:
 Gavin Giovannoni, MBBCh, PhD, FRCP, FRCPath
Gavin Giovannoni, MBBCh, PhD, FRCP, FRCPath
Barts and The London School of Medicine and Dentistry
London, United Kingdom
Peervoice activity supported by educational funding from Biogen International GmbH.
Add comment