- News
- Gene therapy for epilepsy
Gene therapy for epilepsy
 Prof Dimitri Kullmann's team from University College London (UCL) writing in the November 2012 issue of Science Translational Medicine,reports the potential efficacy of gene therapy in focal neocortical epilepsy using a rodent model.
Prof Dimitri Kullmann's team from University College London (UCL) writing in the November 2012 issue of Science Translational Medicine,reports the potential efficacy of gene therapy in focal neocortical epilepsy using a rodent model.
The authors created a rat model of focal neocortical epilepsy where they induced epilepsy by injecting tetanus toxin into the rat motor cortex. In this model the animals show motor seizures and epileptic activity similar to Epilepsia Partialis Continua.
They then injected a lentiviral vector carrying the gene NpHR which encodes for the light-activated chloride ion pump halorhodopsin along with tetanus toxin. A miniaturized wireless system was used to record the EEG activity.A week later, through an optic fiber inserted into the epileptogenic region of the rat brain a laser light in 20-s pulses was delivered to activate halorhodopsin expressed by excitatory principal neurons .When the haloorhodopsin channels are activiated, chloride ions flow into the neurons resulting in membrane hyperpolarization, decreased neuronal excitability and epileptic activity. The controls rats who were not exposed to light did not show any activation of halorhodopsin no suppression of epileptic activity.
In another method using gene therapy they injected a lentiviral vector carrying the gene KCNA1 which encodes Kv1.1 the potassium ion channel.Kv1.1 expression results in an outward flow of potassoim ions, resulting in membrane hyperpolarisation and reduced neuronal excitability.In this situation there was no need for activation using an external light source. They found that following injection of the virus carryin the KCNA1 gene, the animals who have been injected with the tetanus toxin showed a steady reduction in epileptic actiivty. The epileptic activity eventually stopped completely with no resulting motor or behavioural deficits.
Gene therapy approaches has several advantages over drug treatment, in that their action is very specific by delivering the therapeutic gene to a small group of neurons in a specific brain region. Epileptogenic circuits in the brain often results in altered expression of multiple genes resulting in down-regulation of ion channels thereby making AEDs that work by targeting receptors or ion channels less effective.
This study is proof-of-concept that gene therapy can have disease-modifying effects in epilepsy. However this is still in a very early phase of research, and the challenge will be to translate these results into effective treatment for epilepsy.
The optogenetics approach,is disadvantageous in that it necessitates insertion of an optic fiber to deliver the laserlight which activates the injected gene. Besides there are safety issues in inducing the expression of a foreign protein like halorhodopsin. It is also not known for how long halorhodopsin delivered by gene therapy will be expressed by excitatory neurons and whether there are any side effects from long term expression of the halorhodopsin.
At present it is not certain whether viral transfection does not cause any long term risk . It is also not known whether the expression of Kv1.1 channels by principal neurons diminish over time.
This approach also needs to be validated in other animal models of epilepsy, such as temporal lobe epilepsy(TLE), since TLE invovles multiple epileptogenic regions and would require multiple injections of the viral vector. These advances are in the very early stages still and many questions remain unanswered, but certainly opens up exciting prospects for the future.
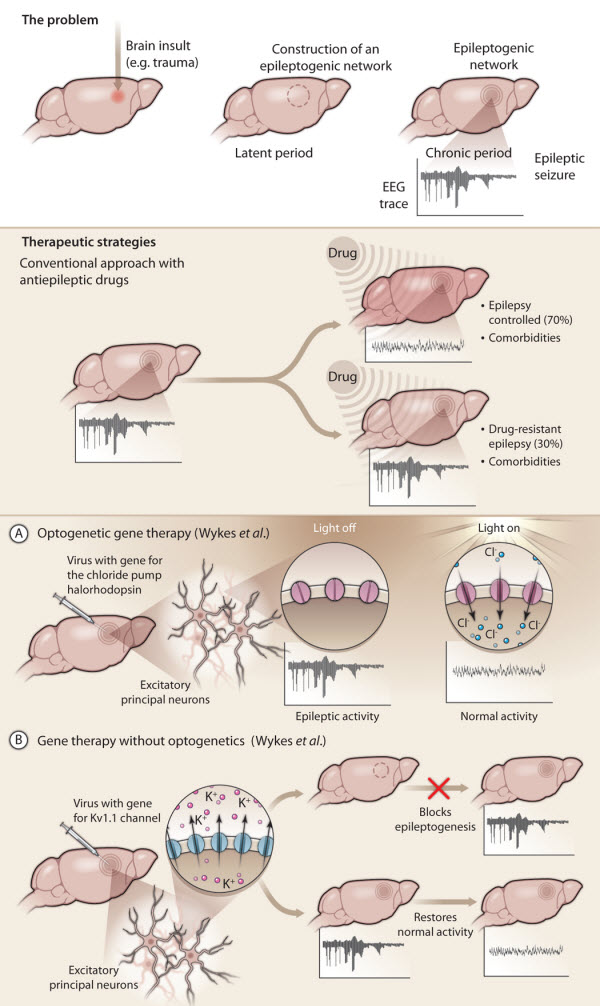
Fig. 1. Gene therapy for treating epilepsy.
(Top) Acquired epilepsies (in contrast to inherited epilepsies) may occur after an insult to the brain, such as trauma or meningitis. In response to the insult, specific neuronal networks in the brain become reorganized (hashed circle) in a process called epileptogenesis. Epileptic seizures are not yet apparent and this latent period can last for decades. At some point, the reorganized networks start to generate spontaneous seizures and full-blown epilepsy emerges (chronic period).
(Middle) Current antiepileptic drugs have many limitations. These drugs only control seizures in 70% of patients, with 30% of patients remaining refractory to drug treatment. None of the current antiepileptic drugs prevent the formation of epileptogenic networks and they often have severe side effects because they act nonspecifically in the brain.
(Bottom) Gene therapy for treating epilepsy specifically targets the affected brain region. In new work, Wykes et al.use two different gene therapy approaches to treat focal epilepsy induced in rat brain by injection of tetanus toxin into the motor cortex.
(A) Using an optogenetics approach, these investigators injected a viral vector carrying the gene for the light-sensitive chloride pump halorhodopsin (pink channels) together with tetanus toxin into rat brain. They used laser light delivered through an optic fiber to activate halorhodopsin expressed by excitatory principal neurons in the epileptogenic region. Activated halorhodopsin allowed chloride ions to flow into the neurons, membrane hyperpolarization, and a decrease in neuronal excitability leading to a reduction in epileptic activity.
(B) The second gene therapy approach involved injecting a viral vector carrying a gene encoding the native potassium ion channel Kv1.1 into rat brain. Overexpression of Kv1.1 (blue channels) by rat excitatory principal neurons resulted in an outward flow of potassium ions, increased membrane hyperpolarization, and decreased neuronal excitability. When virus with Kv1.1 was injected into rat brain at the same time as tetanus toxin, epileptogenesis was prevented.
When virus with Kv1.1 was injected into rat brain after epilepsy had been established, epileptic activity was progressively suppressed over several weeks, ultimately resulting in a normal EEG.
CREDIT: Y. HAMMOND/SCIENCE TRANSLATIONAL MEDICINE
Citations:
Wykes RC, Heeroma JH, Mantoan L, Zheng K, Macdonald DC, Deisseroth K et al. (2012) Optogenetic and Potassium Channel Gene Therapy in a Rodent Model of Focal Neocortical Epilepsy. Sci Transl Med ():. DOI: 10.1126/scitranslmed.3004190 PMID: 23147003.
Read More