- News
- Transplanted Neural Stem Cells shown to produce Myelin
Transplanted Neural Stem Cells shown to produce Myelin
 A Phase I clinical trial led by investigators from the University of California, San Francisco (UCSF) has shown that neural stem cells successfully engrafted into the brains of patients and appear to have produced myelin. The findings have been published in the Oct 10, 2012 issue of Science Translational Medicine.
A Phase I clinical trial led by investigators from the University of California, San Francisco (UCSF) has shown that neural stem cells successfully engrafted into the brains of patients and appear to have produced myelin. The findings have been published in the Oct 10, 2012 issue of Science Translational Medicine.
Once transplanted and engrafted, neural stem cells have the potential to differentiate into a number of different brain cell types, depending on the area of the brain into which they are inserted. The sites chosen for the Phase I study were known from animal studies to be the most likely to result in the formation of oligodendrocytes.
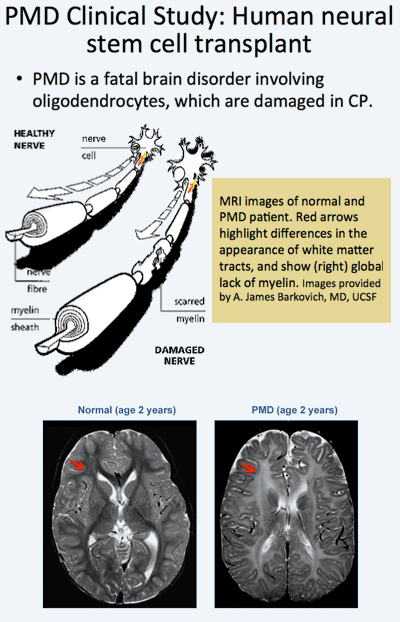 In the trial, allogeneic human neural stem cells (HuCNS-SCs) developed by Stem Cells, Inc., of Newark, California, were injected directly into the frontal lobe white matter of four young children with an early-onset, fatal form of a condition known as Pelizaeus-Merzbacher disease (PMD).
In the trial, allogeneic human neural stem cells (HuCNS-SCs) developed by Stem Cells, Inc., of Newark, California, were injected directly into the frontal lobe white matter of four young children with an early-onset, fatal form of a condition known as Pelizaeus-Merzbacher disease (PMD).
Immunosuppression was administered for 9 months. During 2010-2011, the children with PMD, who were included in the trial, underwent serial neurological evaluations, developmental assessments, and cranial magnetic resonance imaging (MRI) and MR spectroscopy, including high-angular resolution diffusion tensor imaging (DTI). The investigators found evidence that the stem cells had successfully engrafted, receiving blood and nutrients from the surrounding tissue and integrating into the brain.
The Phase 1 trials designed to test safety and preliminary efficacy demonstrated that the neural stem cells were safe in the patients’ brains one year post transplant. No clinical or radiological adverse effects were directly attributed to the donor cells. Reduced T1 and T2 relaxation times were observed in the regions of transplantation 9 months after the procedure in the three subjects. Normalized DTI showed increasing fractional anisotropy and reduced radial diffusivity, consistent with myelination, in the region of transplantation compared to control white matter regions remote to the transplant sites. The MRI findings suggested myelination in the regions that have been transplanted providing indirect evidence that the stem cells had become oligodendrocytes and were producing myelin.
 These results have thus provided for the first time evidence that transplanted neural stem cells are able to produce new myelin in patients with a severe myelination disease. The MRI findings from the clinical trial are further supported by findings from a separate study2 by in a separate study by researchers at Oregon Health & Science University's Papé Family Pediatric Research Institute which showed that neural stem cells injected into mouse models became oligodendrocytes and formed myelin.
These results have thus provided for the first time evidence that transplanted neural stem cells are able to produce new myelin in patients with a severe myelination disease. The MRI findings from the clinical trial are further supported by findings from a separate study2 by in a separate study by researchers at Oregon Health & Science University's Papé Family Pediatric Research Institute which showed that neural stem cells injected into mouse models became oligodendrocytes and formed myelin.
 The PMD clinical trial researchers included Principal investigator David H. Rowitch, MD, PhD, a professor of pediatrics and neurological surgery at UCSF, chief of neonatology at UCSF Benioff Children's Hospital and a Howard Hughes Medical Institute Investigator and co-principal investigator Nalin Gupta, MD, PhD, associate professor of neurological surgery and pediatrics and chief of pediatric neurological surgery at UCSF Benioff Children's Hospital.
The PMD clinical trial researchers included Principal investigator David H. Rowitch, MD, PhD, a professor of pediatrics and neurological surgery at UCSF, chief of neonatology at UCSF Benioff Children's Hospital and a Howard Hughes Medical Institute Investigator and co-principal investigator Nalin Gupta, MD, PhD, associate professor of neurological surgery and pediatrics and chief of pediatric neurological surgery at UCSF Benioff Children's Hospital.
The study, one of the first neural stem cell trials ever conducted in the United States, is symbolical of UCSF’s pioneering role in the stem cell field.
![]() In 1981, Gail Martin, PhD, professor of anatomy, co-discovered embryonic stem cells in mice.
In 1981, Gail Martin, PhD, professor of anatomy, co-discovered embryonic stem cells in mice.
In 2001, Roger Pedersen, PhD, professor emeritus of obstetrics, gynaecology and reproductive sciences, derived two of the first human embryonic stem cell lines.
![]() In 2012, Shinya Yamanaka, MD, PhD, of the UCSF-affiliated Gladstone Institutes and Kyoto University, received the Nobel Prize in Physiology or Medicine for his discovery that adult cells can be reprogrammed to behave like embryonic stem cells
In 2012, Shinya Yamanaka, MD, PhD, of the UCSF-affiliated Gladstone Institutes and Kyoto University, received the Nobel Prize in Physiology or Medicine for his discovery that adult cells can be reprogrammed to behave like embryonic stem cells
Citations
1Gupta N, Henry RG, Strober J, Kang SM, Lim DA, Bucci M et al. (2012) Neural stem cell engraftment and myelination in the human brain. Sci Transl Med 4 (155):155ra137. DOI: 10.1126/scitranslmed.3004373 PMID: 23052294.
2Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR et al. (2012) Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med 4 (155):155ra136. DOI: 10.1126/scitranslmed.3004371 PMID: 23052293.
About Pelizaeus-Merzbacher disease (PMD)
- Rare congenital X-linked recessive leukodystrophy
- Incidence of 1:200,000 to 1:500,000
- Caused by mutation of myelin protein proteolipid protein 1 (PLP1), resulting in hypomyelination
- Leading to death between ages 10 and 15
- Oligodendrocytes are unable to myelinate axons, resulting in loss of normal axonal conduction and neurological dysfunction in the short term, eventually leading to axonopathy and neurodegeneration.
- PMD is one of a spectrum of diseases associated with PLP1, which also includes Spastic Paraplegia Type 2 (SPG2)
- Four types recognised
- Congenital PMD -early-onset severe form of PMD presents with profound neurodevelopmental deficits.
- Classic PMD, in which the early symptoms include muscle weakness, involuntary movements of the eyes (nystagmus), and delays in motor development within the first year of life;
- Complicated SPG2, which features motor development issues and brain involvement, and,
- Pure SPG2, which includes cases of PMD that do not have neurologic complications.
Further Reading
Pelizaeus-Merzbacher disease (PMD)
Read More