An important work published in the May 24 issue of Neuron by, researchers from Dana-Farber Cancer Institute and Harvard Medical School helps toward the understanding of the molecular mechanisms of mitochondrial and metabolic control of seizures and helps to uncover how the ketogenic diet works in epilepsy.
The efficacy of the ketogenic diet in children with epilepsy has pointed to a role for metabolism as a component of the pathogenesis of seizures. However the molecular mechanisms behind the dependence of neuronal activity on metabolic substrates has not been fully understood2. In parallel to its role in apoptosis, the Bcl-2-associated death promoter (BAD) protein , a pro-apoptotic member of the Bcl-2 gene family, has been shown to have an important role in glucose metabolism and utilization.
In this respect the phosphorylation of BAD does not only prevent initiation of cell death, but it is also required for efficient mitochondrial utilization of glucose in liver. Dephosphorylated BAD forms a heterodimer with Bcl-2 and Bcl-xL, inactivating them and thus allowing Bax/Bak-triggered apoptosis.
Giménez-Cassina et al. (2012) investigating the potential role of BAD in seizures, found the existence of a phosphodependent regulatory switch in BAD that reduces neuronal excitability upon kainic acid-induced seizures. Similar to what occurs in the liver they showed that cortical neurons and astrocytes from Bad−/− mice display lower glucose utilization for mitochondrial respiration.
This showed an interesting connection between BAD deficient mice or mice with nonphosphorylable BAD and ketogenic diet which reduces seizures in terms of glucose utilization by mitochondria.
The authors therefore investigated whether BAD might also influence seizure sensitivity in vivo. They found that BAD deficient as well as BadS155A(mice bearing a phosphodeficient knockin allele of Bad at serine 155) mice were significantly protected from the proconvulsant drug kainic acid. Moreover, seizure resistance was specific for BAD and independent from its proapoptotic function, pointing therefore to its role in metabolism.
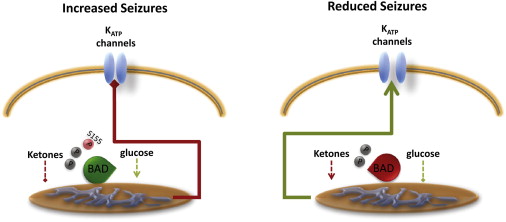 BAD Phosphorylation Controls Mitochondrial Fuel Utilization to Switch Neuronal ExcitabilityThe proapoptotic BH3-only protein BAD can be viewed as a relay: its phosphorylation on Ser 155 switches the preferred source of reducing equivalents for mitochondria from glucose to ketones. Plasma membrane KATP channels are closed in neurons with “glycolytic” mitochondria, as opposed to the ones where mitochondria use ketone bodies and that are less susceptible to seizures. (source: Neuron Volume 74, Issue 4, 24 May 2012, Pages 600–602)The Ketogenic diet has been shown to increases the activity of KATP channels. KATP channels are activated following decreased intracellular ATP, in a negative feedback loop that is believed to help neurons to overcome excitotoxicity during seizure. Giménez-Cassina et al. also found that the dentate granule neurons (DGNs) of hippocampal slices from BAD deficient (Bad−/− ) mice showed increased single KATP channels, while ablation of KATP channels expression in BAD deficient (Bad−/− ) mice diminished their resistance to seizures .
BAD Phosphorylation Controls Mitochondrial Fuel Utilization to Switch Neuronal ExcitabilityThe proapoptotic BH3-only protein BAD can be viewed as a relay: its phosphorylation on Ser 155 switches the preferred source of reducing equivalents for mitochondria from glucose to ketones. Plasma membrane KATP channels are closed in neurons with “glycolytic” mitochondria, as opposed to the ones where mitochondria use ketone bodies and that are less susceptible to seizures. (source: Neuron Volume 74, Issue 4, 24 May 2012, Pages 600–602)The Ketogenic diet has been shown to increases the activity of KATP channels. KATP channels are activated following decreased intracellular ATP, in a negative feedback loop that is believed to help neurons to overcome excitotoxicity during seizure. Giménez-Cassina et al. also found that the dentate granule neurons (DGNs) of hippocampal slices from BAD deficient (Bad−/− ) mice showed increased single KATP channels, while ablation of KATP channels expression in BAD deficient (Bad−/− ) mice diminished their resistance to seizures .
The study thus linked BAD phosphorylation and KATP channels activity to the attenuation of seizures, a hitherto unknown signalling pathway The study, funded by Harvard Catalyst, Citizens United for Research in Epilepsy, and the NIH has contributed significantly to the understanding of the molecular mechanisms of mitochondrial and metabolic control of seizures with potential implications for future therapeutic strategies.
Citations
1. Elena Ziviani, Luca Scorrano. Neuron, Volume 74, Issue 4, 24 May 2012, Pages 600-602
2. Giménez-Cassina et al. Neuron, 74 (2012), pp. 719–730
Read More