Lenmeldy (atidarsagene autotemcel) has received approval from the U.S. Food and Drug Administration. This marks a significant milestone as it is the first gene therapy to be approved by the FDA for the treatment of children with pre-symptomatic late infantile, pre-symptomatic early juvenile or early symptomatic early juvenile metachromatic leukodystrophy (MLD).
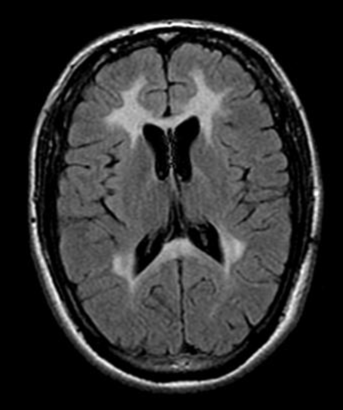
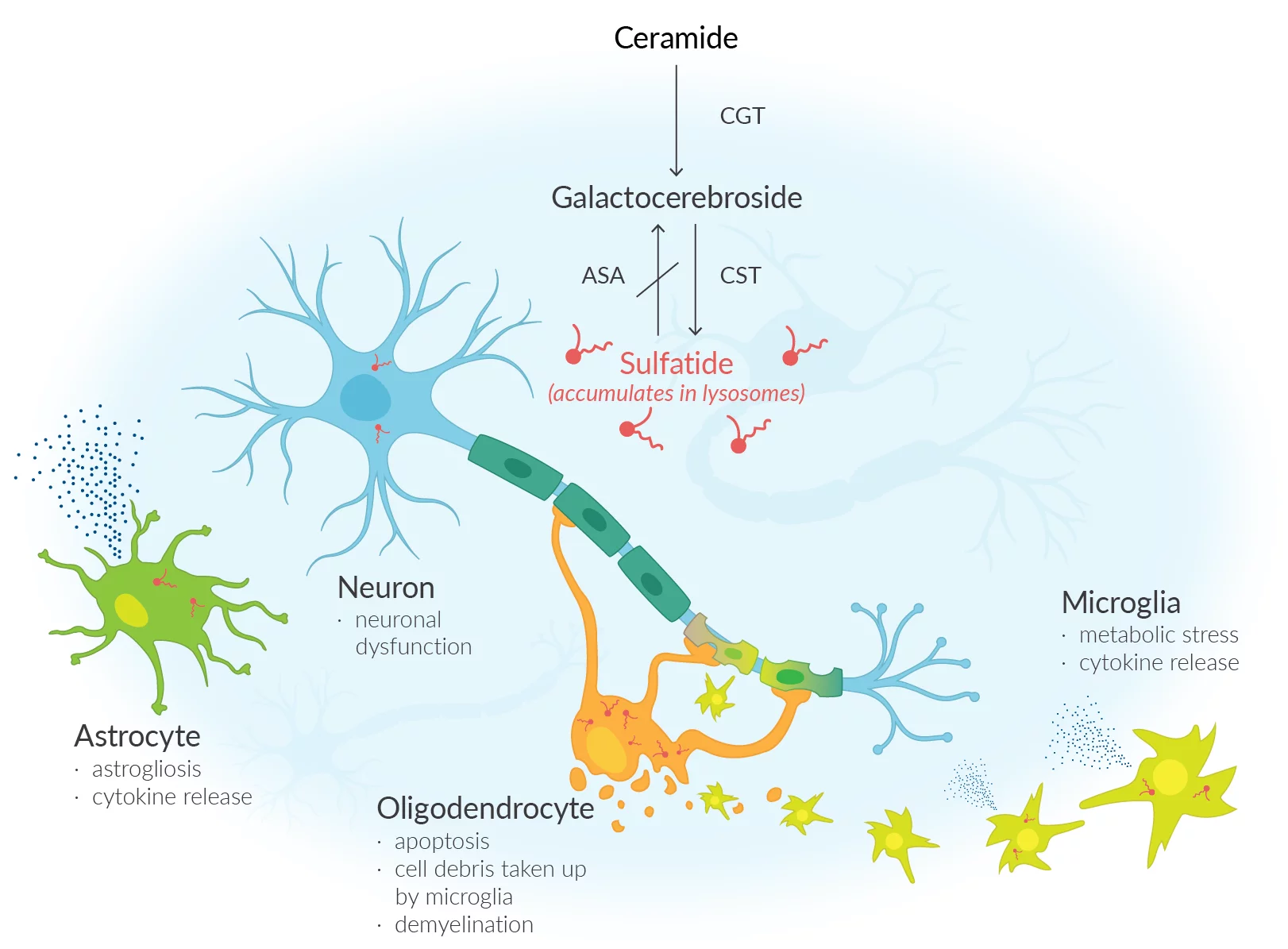
Metachromatic leukodystrophy a debilitating rare condition arises due to a lack of an enzyme known as arylsulfatase A (ARSA), resulting in an accumulation of sulfatides (fatty substances) within the cells. The accumulation of this substance results in damage to both the central and peripheral nervous systems, resulting in the decline of motor and cognitive abilities and ultimately premature mortality. Approximately one in every 40,000 individuals in the United States is affected by MLD. Unfortunately, MLD does not currently have a cure. The main approach to treatment involves providing support and managing symptoms.
Lenmeldy is a unique treatment that involves a personalized infusion of the patient's own hematopoietic (blood) stem cells. These cells have been genetically modified to include functional copies of the ARSA gene. The stem cells are obtained from the patient and modified by incorporating a functional copy of the ARSA gene. The modified stem cells are transplanted back into the patient, where they attach and multiply within the bone marrow. The modified stem cells provide the body with myeloid (immune) cells that generate the ARSA enzyme, aiding in the breakdown of sulfatides and potentially halting the advancement of MLD. Before treatment, patients are required to undergo high-dose chemotherapy, which involves the removal of cells from the bone marrow to make way for the modified cells in Lenmeldy.
The safety and effectiveness of Lenmeldy was evaluated using data from 37 children who participated in two single-arm, open-label clinical trials and an expanded access program. Children who underwent treatment with Lenmeldy were compared to a group of untreated children, allowing for a comparison of their progress over time. The main measure of effectiveness was the duration of time without severe motor impairment, which was defined as the period from birth until the first instance of losing the ability to move and sit without assistance, or until death. In children diagnosed with MLD, the administration of Lenmeldy has shown significant effectiveness in reducing the likelihood of severe motor impairment or mortality when compared to children who did not receive treatment. At 6 years of age, all children who received treatment with Lenmeldy were still alive, in contrast to only 58% of children in the natural history group. By the age of 5, a significant majority of children who received treatment were able to walk without any help. The majority of the children who received treatment showed normal language and performance IQ scores, a finding that has not been observed in children who did not receive treatment. Furthermore, young patients who exhibited pre-symptomatic early juvenile and early symptomatic early juvenile MLD experienced a decline in motor and/or cognitive function.
Some potential side effects of Lenmeldy include fever, low white blood cell count, mouth sores, respiratory infections, rash, medical line infections, viral infections, gastrointestinal infections, and enlarged liver.
Following the administration of Lenmeldy, it is important to closely observe patients for any changes in neutrophil counts and the potential for delayed platelet engraftment until engraftment has been successfully accomplished. There are potential risks associated with Lenmeldy treatment, including the possibility of blood clot formation or the development of encephalitis, a swelling of the brain tissues. Although there is a potential risk of blood cancer, it is important to note that no cases have been observed in patients who have undergone treatment with Lenmeldy. It is important for patients to undergo regular monitoring for hematologic malignancies, which includes an annual complete blood count (with differential). Integration site analysis should also be considered, as needed, for a minimum of 15 years following treatment.
Add comment