 Evidence seems to be accumulating which suggests that Coronavirus 2 (SARS-CoV-2) could affect the central nervous system and might contribute to the respiratory failure seen in these patients.
Evidence seems to be accumulating which suggests that Coronavirus 2 (SARS-CoV-2) could affect the central nervous system and might contribute to the respiratory failure seen in these patients.
Mao et al. [2020] in a retrospective case series from Wuhan, China looked at two hundred fourteen hospitalized patients with laboratory confirmed diagnosis of severe acute respiratory syndrome from coronavirus 2 (SARS-CoV-2) infection. They found that neurological symptoms were seen in severely affected patients and manifested as acute stroke (6%), consciousness impairment (15%), and skeletal muscle injury (19%).
Recently Zhao and colleagues from China [Zhao et al, 2020] reported the case of a 61yrs old woman who presented with acute weakness in both legs and severe fatigue with the symptoms worsening over 24 hours. The woman returned from Wuhan 4days earlier but had not exhibited any symptoms of COVID-19 on admission.She was diagnosed with Guillain-Barré syndrome and given intravenous immunoglobulin. On day 8 of admission, however she developed a dry cough and high fever and oropharyngeal swabs taken were positive for SARS-CoV-2. In retrospect although asymptomatic, her laboratory findings on admission of lymphocytopenia and thrombocytopenia were consistent with COVID-19 suggesting the presence of SARS-CoV-2 infection. The temporal association led the authors to speculate that peculate that SARS-CoV-2 infection might have been responsible for the development of Guillain-Barré syndrome.In addition the symptoms of Guillain-Barré syndrome overlapped with that of SARS-CoV-2 suggesting a parainfectious phenomenon.
Filatov et al [2020] from Charles E. Schmidt College of Medicine, USA recently reported on a 74-year-old patient who travelled from Europe to the United States and presented with encephalopathy and was found to be COVID-19 positive.
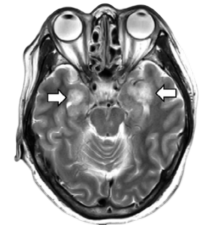
A recent report in Radiology [Poyiadji et al., 2020] described a female airline worker in her late fifties who presented with a 3-day history of cough, fever, and altered mental status.She was subsequently confirmed positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and developed acute necrotizing hemorrhagic encephalopathy with characteristic radiological findings. Images from brain MRI demonstrated hemorrhagic rim enhancing lesions within the bilateral thalami, medial temporal lobes, and subinsular regions.
COVID-19 shares highly homological sequence with SARS‐CoV, and causes acute, severe pneumonia with clinical symptoms similar to those reported for SARS‐CoV and MERS‐CoV. During the 2003 SARS epidemic patients with severe acute respiratory syndrome (SARS) were noted to have central nervous symptoms during the course of illness. Studies that looked at samples from patients with classic SARS following the outbreak , had found virus particles inside neurons, including in brainstem respiratory centers.
Researchers from Guangzhou, People’s Republic of China in 2015 isolated a SARS coronavirus strain from a brain tissue specimen obtained from a patient with SARS with significant central nervous symptoms [Xu et al., 2015]. This study had provided direct evidence that SARS human coronavirus is capable of infecting the central nervous system, and that B lymphocyte antigen receptors (mIg) might be involved in the brain immunopathology of SARS. The neurotropic, neuroinvasive and neurovirulent characteristics of Coronovirus has already been suggested previously by other authors [Compton et al., 1993] [Hensley et al., 1998] [Arbour et al., 2000].
Access to the CNS by coronavirus is thought be via the olfactory nerve, haematogenous route or via lymphatics [Perlman et al., 1990] [Barnett et al., 1993] [Barthold et al., 1992]. Coronaviruses could pass through synaptic pathways from the mechanoreceptors and chemoreceptors in the lung and lower respiratory airways to brainstem cardiorespiratory centers. Since there is considerable similarity between SARS‐CoV and SARS‐CoV2, it is possible that CNS invasion by SARS‐CoV2 could have contributed to the acute respiratory failure seen in these patients.
Public Health England has also alerted health professionals to recent reports from affected patients of loss of smell as a symptom of COVID-19 infection. Post-viral anosmia is one of the leading causes of loss of sense of smell in adults accounting for about 40% of cases. Previously described coronaviruses are thought to account for 10-15% cases. Hence it is entirely plausible that the anosmia could be seen with the novel COVID-19 virus. Other countries including Iran, China, Italy and South Korea have also reported anosmia/hyposmia in COVID-19 patients.
ENTUK , the professional membership body representing Ear, Nose and Throat surgery, as well as its related specialities, in the United Kingdom has suggested that although some of the patients reporting anosmia or hyposmia are otherwise well and may not meet current criteria for testing or selfisolation, it could potentially be used as a screening tool to help identify otherwise asymptomatic patients and be advised to self isolate. They have also advised against the use of oral steroids in the treatment of new onset anosmia during the pandemic [Hopkins & Kumar, 2020].
The invasion of the respiratory system by COVID-19 is mediated via ACE-2 receptor. ACE2, is also expressed in the small intestine, the kidneys, the pancreas, and the lining of blood capillaries. Although ACE2 was thought not to be highly expressed in the brain there is evidence to suggest that ACE2 expression is quite ubiquitous and is present in a wide variety of tissues, including the brain [Igase et al., 2005] [Sakima et al., 2005] [Doobay et al., 2007]. Although not confirmed the current hypothesis is that given the similarity between classic SARS and COVID-19, it can potentially cause respiratory failure by affecting the brainstem respiratory centers.
References
Arbour N, Day R, Newcombe J, Talbot PJ (2000) Neuroinvasion by human respiratory coronaviruses. J Virol 74 (19):8913-21. DOI: 10.1128/jvi.74.19.8913-8921.2000 PMID: 10982334.
Barnett EM, Perlman S (1993) The olfactory nerve and not the trigeminal nerve is the major site of CNS entry for mouse hepatitis virus, strain JHM. Virology 194 (1):185-91. DOI: 10.1006/viro.1993.1248 PMID: 8386871.
Barthold SW, Smith AL (1992) Viremic dissemination of mouse hepatitis virus-JHM following intranasal inoculation of mice. Arch Virol 122 (1-2):35-44. DOI: 10.1007/bf01321116 PMID: 1309644.
Compton SR, Barthold SW, Smith AL (1993) The cellular and molecular pathogenesis of coronaviruses.Lab Anim Sci 43 (1):15-28. PMID: 8384676.
Doobay MF, Talman LS, Obr TD, Tian X, Davisson RL, Lazartigues E (2007) Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system.Am J Physiol Regul Integr Comp Physiol 292 (1):R373-81. DOI: 10.1152/ajpregu.00292.2006 PMID: 16946085.
Filatov A, Sharma P, Hindi F, et al. (March 21, 2020) Neurological Complications of Coronavirus Disease (COVID-19): Encephalopathy. Cureus 12(3): e7352. DOI 10.7759/cureus.7352
Hensley LE, Holmes KV, Beauchemin N, Baric RS (1998) Virus-receptor interactions and interspecies transfer of a mouse hepatitis virus.Adv Exp Med Biol 440 ():33-41. DOI: 10.1007/978-1-4615-5331-1_5 PMID: 9782262.
Hopkins, C., & Kumar, N. ENTUK Royal College of Surgeons of England (2020). Loss of sense of smell as marker of COVID-19 infection. Retrieved from https://www.entuk.org/sites/default/files/files/Loss of sense of smell as marker of COVID.pdf
Igase M, Strawn WB, Gallagher PE, Geary RL, Ferrario CM (2005) Angiotensin II AT1 receptors regulate ACE2 and angiotensin-(1-7) expression in the aorta of spontaneously hypertensive rats.Am J Physiol Heart Circ Physiol 289 (3):H1013-9. DOI: 10.1152/ajpheart.00068.2005 PMID: 15833808.
Ling Mao, Mengdie Wang, Shanghai Chen, Quanwei He, Jiang Chang, Candong Hong, Yifan Zhou, David Wang, Yanan Li, Huijuan Jin, Bo Hu. Neurological Manifestations of Hospitalized Patients with COVID-19 in Wuhan, China: a retrospective case series study. Accessed March 21, 2020. https://www.medrxiv.org/content/10.1101/2020.02.22.20026500v1 doi: https://doi.org/10.1101/2020.02.22.20026500
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients.J Med Virol ():. DOI: 10.1002/jmv.25728 PMID: 32104915.
Perlman S, Evans G, Afifi A (1990) Effect of olfactory bulb ablation on spread of a neurotropic coronavirus into the mouse brain. J Exp Med 172 (4):1127-32. DOI: 10.1084/jem.172.4.1127 PMID: 1698910.
Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B (2020) COVID-19-associated Acute Hemorrhagic Necrotizing Encephalopathy: CT and MRI Features. Radiology ():201187. DOI: 10.1148/radiol.2020201187 PMID: 32228363.
Sakima A, Averill DB, Gallagher PE, Kasper SO, Tommasi EN, Ferrario CM | display-authors=etal (2005) Impaired heart rate baroreflex in older rats: role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii.Hypertension 46 (2):333-40. DOI: 10.1161/01.HYP.0000178157.70142.33 PMID: 16009784.
Xu J, Zhong S, Liu J, Li L, Li Y, Wu X | display-authors=etal (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41 (8):1089-96. DOI: 10.1086/444461 PMID: 16163626.
Zhao H, Shen D, Zhou H, Liu J, Chen S (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol ():. DOI: 10.1016/S1474-4422(20)30109-5 PMID: 32246917.
Image source: from Poyiadji et al., 2020. MRI images demonstrate T2 FLAIR hyperintensity within the bilateral medial temporal lobes