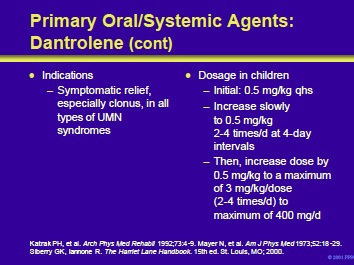
Dantrolene is indicated for the symptomatic relief of the clinical manifestations of spasticity, especially clonus. It is indicated for spasticity in patients with all types of upper motor neuron syndromes and is particularly useful in patients with symptoms from severe hypoxic brain injury. The drug is supplied as 25-, 50-, and 100-mg capsules and in 20 mg vials for reconstitution and injection.
Children should be started on a dosage of 0.5 mg/kg qhs and increased slowly to 2 to 4 times a day at 4-day intervals. The dosage can then be increased by 0.5 mg/kg to a maximum of 3 mg/kg/dose, administered two to four times a day.
The maximum dose should not exceed 400 mg/d. Adults should be started on a 25 mg dose qhs and slowly increased to a maximum of 100 mg qid.
References:
Katrak PH, Cole AM, Poulous CJ, et al. Objective assessment of spasticity, strength, and function with early exhibition of dantrolene sodium after cerebrovascular accident: a randomized double-blind study. Arch Phys Med Rehabil 1992;73:4-9.
Mayer N, Mecomber SA, Herman R. Treatment of spasticity with dantrolene sodium. Am J Phys Med 1973;52:18-29.
Siberry GK, Iannone R. The Harriet Lane Handbook . 15th ed. St. Louis, MO; 2000.
Article Index
- 5. Oral Medications for the Treatment of Spasticity
- Physiologic Basis of Antispasticity Medications
- Spinal Effector Mechanisms
- Myotactic Reflex
- Primary and Secondary Antispasticity Drugs
- Primary Oral/Systemic Agents: Diazepam
- Primary Oral/Systemic Agents: Diazepam (cont)
- Primary Oral/Systemic Agents: Diazepam (cont)
- Primary Oral/Systemic Agents: Baclofen
- Primary Oral/Systemic Agents: Baclofen (cont)
- Primary Oral/Systemic Agents: Baclofen (cont)
- Primary Oral/Systemic Agents: Dantrolene
- Primary Oral/Systemic Agents: Dantrolene (cont)
- Primary Oral/Systemic Agents: Dantrolene (cont)
- Primary Oral/Systemic Agents: Tizanidine
- Primary Oral/Systemic Agents: Tizanidine (cont)
- Primary Oral/Systemic Agents: Tizanidine (cont)
- Secondary Oral/Systemic Agents
- All Pages
Page 13 of 18

