- Webinars
- 5. Oral Medications for the Treatment of Spasticity
5. Oral Medications for the Treatment of Spasticity
Hot

The following sections expand upon the various treatment options in patients with spasticity secondary to cerebral palsy.
They include:
- Pharmacotherapy
- Surgical therapy
- Rehabilitation
Antispasticity medications exert their effects upon motor centers including:
- Higher centers in the cortex, basal ganglia, and cerebellum. Cortical neurons whose influence may be modulated include projection neurons and interneurons. Neurons in the cortex and other motor control regions may utilize a variety of neurotransmitters including glutamate, glutamine, GABA and dopamine.
Descending pathways and nuclei that may be influenced by antispasticity medications include the following:
- Reticulospinal tract: trunk and proximal extremity control with serotonin and norepinephrine as neurotransmitters
- Pyramidal tracts: discrete distal extremity control with glutamate as a neurotransmitter
- Vestibulospinal tract: trunk and head control
- Locus ceruleus: facilitatory drive or spinal cord reflex activity; activity reduced by a2 adrenergic receptor agonists
As shown on the next slide, spinal effector mechanisms are also active.
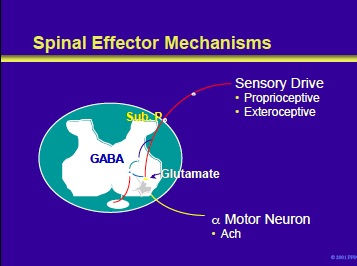
Spinal effector mechanisms include sensomotor reflexes with inputs from proprioceptive (muscle spindles) and exteroceptive (pain [nociceptive], anxiety, infectious processes) and afferent from alpha motor neurons.
The activity of the latter is a summation of excitatory and inhibitory influences with acetylcholine as the excitatory neurotransmitter.
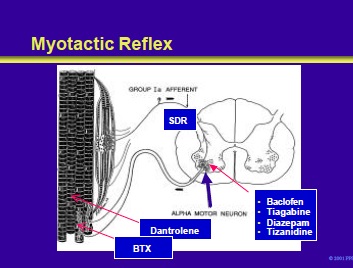
The sense organ for the myotactic reflex is the muscle spindle. Stretch of the muscle spindle stimulates primary Ia afferents that enter the cord through the sensory dorsal roots.
These Ia fibers synapse directly on the alpha motor neurons that fire and cause the stretched muscle to contract. The resistance of a muscle to stretch is called tone. Transection of the motor nerves to muscle produces flaccidity.
Hyperactive stretch reflexes result in a hypertonic or spastic muscle. This slide illustrates the site of action of various drugs (discussed in the following slides) used in the treatment of spasticity.

As shown in this slide, antispasticity drugs (ASDs) can be divided into primary and secondary groups of medications. Primary ASDs are the first-line agents in the treatment of spasticity.
Patients with resistant or refractory spasticity, or who develop adverse reactions to primary agents, may respond to a secondary ASD. The mechanisms and anatomic sites of actions of the ASDs are not completely understood.
However, they are believed to either alter the function of neurotransmitters or modulators in the central and/or peripheral nervous system. The CNS actions could include suppression of excitation via glutamate, enhancement of inhibition via GABA or glycine, or both.
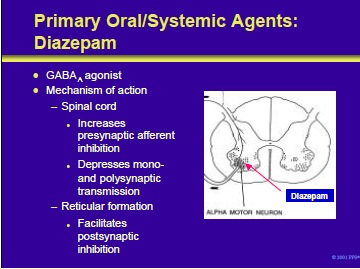
Diazepam and other benzodiazepines exert their antispastic activities through the benzodiazepine-GABAA receptor complex. This is an inotropic effect mediated through the chloride channel.
Mechanisms of action of these compounds include the following:
- Enhancement of presynaptic inhibition at the spinal cord level
- Inhibition of monosynaptic and polysynaptic spinal cord reflexes
- Facilitation of post-synaptic inhibition by the reticular system within the spinal cord
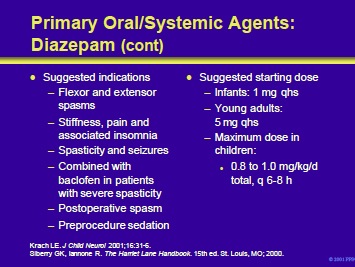
Diazepam and other benzodiazepines can be used for the following indications in the patient with cerebral palsy:
- Flexor and extensor spasms
- Stiffness, pain, and insomnia
- Spasticity and seizures
- In combination with baclofen in patients with severe spasticity
- Postoperative spasm
- Preprocedure sedation
Since diazepam produces CNS depression, it is often prescribed at night to suppress spasms that may produce insomnia. In infants and children, therapy is initiated with 1 to 2 mg at bedtime. In young adults, treatment can begin with 5 mg which can later be increased to 10 mg. The maximum dose for children is 0.8 to 1.0 mg/kg/d in divided doses.

Benzodiazepines have a wide margin of safety. However, they can produce CNS depression with a resultant sedation, respiratory depression and inhibition of cardiovascular function. Paradoxical irritability can occur.
True physiological withdrawal can develop. A withdrawal syndrome has been described and consists of the following: agitation, anxiety, restlessness, tremor, muscle fasciculation and twitching, insomnia, nightmares, seizures, hyperpyrexia and hallucinations.
Paradoxical sleep disorders can also occur. Patients may also experience increased oral secretions. When weaning from this medication, taper over 3 to 6 months.
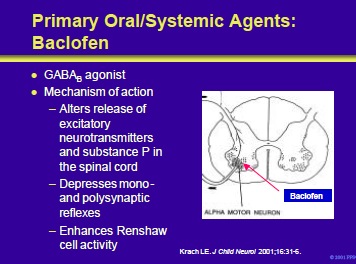
Baclofen is a structural analog of GABA. It appears to be at the level of the spinal cord by binding to GABAB receptors and blocking polysynaptic and monosynaptic afferents. Its mechanism of action may be as a direct inhibitory neurotransmitter or the result of hyperpolarization of afferent nerve terminals.
Krach LE. Pharmacotherapy of spasticity: oral medications and intrathecal baclofen. J Child Neurol 2001;16:31-6.
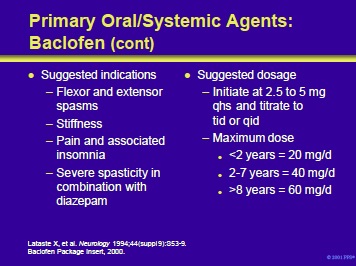
Oral baclofen is useful for symptomatic problems such as flexor and extensor spasms, stiffness and pain in patients with spinal cord injury and demyelinating myelopathies.
In patients with severe spasticity, it can be used in conjunction with diazepam. The efficacy of baclofen in patients with cerebrovascular accidents and cerebral palsy has not been established.
Oral baclofen is available as a refrigerated suspension of 5 mg/mL or as 10- and 20-mg tablets. When refrigerated, the suspension is stable for up to 35 days. Treatment in children is initiated with 2.5 to 5 mg qhs and titrated at weekly intervals to tid or qid dosages. In older patients, initiate therapy with 5 mg bid. Maximum doses for children are shown on the slide. In adults, the maximum dose ranges from 80 to 200 mg/d.
References:
Baclofen Package Insert, 2000.
Lataste X, Emre M, Davis C, et al. Comparative profile of tizanidine in the management of spasticity. Neurology 1994;44(suppl 9):S53-9.
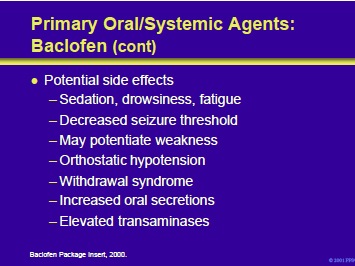
Potential side effects include CNS depression and weakness. The former may manifest as sedation, drowsiness, and fatigue. In patients with epilepsy, baclofen may decrease their seizure threshold. Baclofen-induced weakness may produce a deterioration in the ability of some patients to walk. In addition, orthostatic hypotension may occur.
Patients may also experience increased oral secretions. Elevations in transaminases have been reported and liver function tests should be monitored. The drug should never be stopped suddenly; sudden withdrawal may precipitate a syndrome that includes hallucinations, psychosis and seizures.
Baclofen Package Insert, 2000.
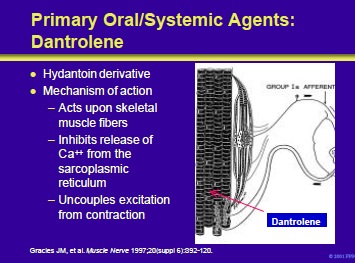
Dantrolene is a lipophilic hydantoin derivative that easily crosses cell membranes and achieves wide tissue distribution. It is unique among antispasticity drugs in that it works at the level of the skeletal muscle. Normally, muscle membrane depolarization causes the release of calcium from the sarcoplasmic reticulum with a resultant contraction.
Dantrolene decreases calcium release. As a result, there is uncoupling of the contractile process and a reduction in the force of contraction. The percentage reduction of force for twitch or low frequency stimulation of muscle is much greater than for high frequency or tetanic stimulation because more calcium is released in the latter.
For unknown reasons, dantrolene has little effect on cardiac and smooth muscle fibers.
Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity part II: general and regional treatments. Muscle Nerve 1997;20(suppl 6):S92-120.
Dantrolene is indicated for the symptomatic relief of the clinical manifestations of spasticity, especially clonus. It is indicated for spasticity in patients with all types of upper motor neuron syndromes and is particularly useful in patients with symptoms from severe hypoxic brain injury. The drug is supplied as 25-, 50-, and 100-mg capsules and in 20 mg vials for reconstitution and injection.
Children should be started on a dosage of 0.5 mg/kg qhs and increased slowly to 2 to 4 times a day at 4-day intervals. The dosage can then be increased by 0.5 mg/kg to a maximum of 3 mg/kg/dose, administered two to four times a day.
The maximum dose should not exceed 400 mg/d. Adults should be started on a 25 mg dose qhs and slowly increased to a maximum of 100 mg qid.
References:
Katrak PH, Cole AM, Poulous CJ, et al. Objective assessment of spasticity, strength, and function with early exhibition of dantrolene sodium after cerebrovascular accident: a randomized double-blind study. Arch Phys Med Rehabil 1992;73:4-9.
Mayer N, Mecomber SA, Herman R. Treatment of spasticity with dantrolene sodium. Am J Phys Med 1973;52:18-29.
Siberry GK, Iannone R. The Harriet Lane Handbook . 15th ed. St. Louis, MO; 2000.

Because of the potential of hepatotoxicity, dantrolene should be administered cautiously in patients with a history of liver disease or those receiving other medications that might compete for its metabolic pathways. In addition, liver function tests should be performed at baseline and periodically (2 to 3 times per year) thereafter.
Potential side effects of dantrolene therapy include muscle weakness, diarrhea, mild drowsiness, paresthesias and dysphagia.
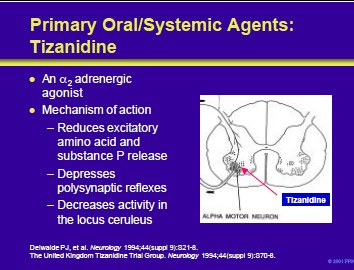
Tizanidine is an imidazole derivative with agonistic action at both spinal and supraspinal a2 adrenergic receptors. It acts to prevent the release of excitatory amino acids and substance P from the presynaptic terminals of spinal interneurons.
Tizanidine may also facilitate the inhibitory actions of glycine, suppress acetylcholine-induced excitation of Renshaw cell activity and exert antinociceptive actions. As an a2 adrenergic agonist, tizanidine slows the firing of neurons in the locus ceruleus.
This leads to inhibition of the facilitatory activity that the brainstem nucleus exerts upon reflex activity in the lumbar portion of the spinal cord.
References:
Delwaide PJ, Pennisi G. Tizanidine and electrophysiologic analysis of spinal control mechanisms in humans with spasticity. Neurology 1994;44(suppl 9):S21-8.
The United Kingdom Tizanidine Trial Group. A double-blind, placebo-controlled trial of tizanidine in the treatment of spasticity caused by multiple sclerosis. Neurology 1994;44(suppl 9):S70-8.

Tizanidine is indicated for nighttime spasms, pain and clonus in patients with cerebral palsy and other upper motor neuron syndromes. The drug is supplied as 2- and 4-mg tablets.
Tablets can be crushed and given in water or administered via a gastrostomy or jejunostomy tube. In children < 10 years of age, treatment should begin at 1 mg qhs and increase at weekly intervals to qid dosing as tolerated. In children >10 years of age, begin at 2 mg qhs and increase at weekly intervals to qid dosing as tolerated.
The target dose is 0.3 to 0.5 mg/kg/d.
Wagstaff AJ, Bryson HM. Tizanidine: a review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs 1997;53:435-52.

Tizanidine side effects include sedation, fatigue, drowsiness, dry mouth, dizziness and orthostatic hypotension. While the latter is less common than that seen with clonidine, the risk is real. Patients requiring this medicine who experience orthostatic hypotension can be treated with a mineralocorticoid such as fludrocortisone acetate. Other side effects include visual hallucinations (3%) and hepatotoxicity (5%).
Because of the risk of liver injury, tizanidine should not be used in conjunction with either dantrolene or valproate as well as other medications that are metabolized through the liver.
The current practice is to monitor LFTs at baseline, 6 weeks, 3 months and 6 months after therapy is started. Some clinicians will then continue to monitor liver function periodically thereafter.
Wagstaff AJ, Bryson HM. Tizanidine: a review of its pharmacology, clinical efficacy and tolerability in the management of spasticity associated with cerebral and spinal disorders. Drugs 1997;53:435-52. Young RR. Spasticity: a review. Neurology 1994:(suppl 9):S12- 20.

Tiagabine is primarily used for its antiepileptic activity but has also been employed in patients with spasticity. The precise mechanism of its action is unknown. However, it is believed to enhance the activity of GABA, the major inhibitory neurotransmitter in the CNS, predominantly by blocking GABA reuptake. Cyproheptadine is a 5-HT antagonist that may neutralize the spinal and supraspinal serotoninergic excitatory inputs.
It has been used in some patients with spasticity. Clonidine is a selective a2-receptor agonist that works at multiple levels. Its antispasticity activity may be due to enhancement of a2-mediated presynaptic inhibition of sensory afferents. Openlabel reports have described efficacy in patients with spasticity. Hypotension is a potential side effect.
Lamotrigine is an antiepileptic drug that blocks voltage and usedependent sodium channels and reduces the release of glutamate and other excitatory amino acids. These actions may decrease the manifestations of spasticity in some patients. Gabapentin is a GABA analogue with antiepileptic properties. It modulates the activity of enzymes that metabolize glutamate and may be useful in some patients with spasticity.
Carbidopa-levodopa is a combination of levodopa, a dopamine precursor, and carbidopa, a peripheral inhibitor of levodopa decarboxylation. This combination may be useful in patients with progressively worsening diplegia.
Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity part II: general and regional treatments. Muscle Nerve 1997;20(suppl 6):S92-S120.
- Next Section : Intrathecal Baclofen for Spasticity in Cerebral Palsy

