Page 32 - ILAE_Lectures_2015
P. 32
from slight rotation to
complete inversion in
relation to the pial
surface81. These cells
show dysmorphic and
extremely tortuous
dendrites but with
decreased spine density.
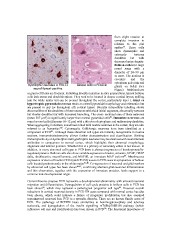
Balloon cells have large
round soma with a
diameter of 2090 m
or more. The nucleus is
eccentric and the
cytoplasm pale pink and
glassy on H&E (see
Figure). Multinucleate
or giant cell forms are frequent. Following biocytin injection in slice preparations, typical balloon
cells lack axons and dendritic spines. They tend to be located in deeper cortical layers, spilling
into the white matter but can be present throughout the cortex, particularly layer I. Giant or
hypertrophic pyramidal neurones retain an overall pyramidal morphology and orientation but
are present in any (or throughout all) cortical layers. Biocytin intracellular labelling shows
abnormalities of the dendrites of these neurones with thick initial segments, abnormally tortuous
but shorter dendrites but with increased branching. The cross- sectional area of these neurones
(mean 507 µm2) is significantly larger than normal pyramidal cells81. Immature neurones are
round or oval cells (diameter 1012 µm) with a thin rim of cytoplasm and rudimentary dendrites.
When aggregating in clusters, sometimes mixed with mature neurones in the cortex, they are also
referred to as ‘hamartias’82. Cytomegalic GABAergic neurones have been identified as a
component of FCD83. Although these abnormal cell types are instantly recognisable in routine
sections, immumohistochemistry allows further characterisation and classification. Striking
immunopositivity of dysmorphic and hypertrophic neurones may be observed with neurofilament
antibodies in comparison to normal cortex, which highlights their abnormal morphology,
alignment and laminar position. Whether this is a primary or secondary effect is not known. In
addition, in many abnormal cell types in FCD there is aberrant expression of developmentally-
regulated proteins. Balloon cells also show variable expression of nestin, vimentin, GFAP, GFAP
delta, doublecortin, neurofilaments, and MAP1B, an immature MAP isoform84. Membranous
expression of stem cell marker CD34 (and CD133) is seen in FCD cases in a proportion of balloon
cells located predominantly in the white matter85,86. Co-expression of neuronal and glial markers
by abnormal cell types has been shown84,87, confirming aberrant glial-neuronal differentiation
and this observation, together with the expression of immature proteins, lends support to a
common mal-developmental origin.
Current theories propose FCD represents a developmental abnormality with arrested neuronal
migration and differentiation. Dysregulation of cell cycle proteins in balloon cells in FCD has
been shown88, which may represent a pathological progenitor cell type89. However overall
reductions in cortical neuronal density in FCD IIB cases compared with normal cortex has also
been shown, which could indicate a failure of progenitor proliferation but also acquired
superimposed neuronal loss. FCD is a sporadic disorder. There are no known family cases of
FCD. The pathology of FCDIIB bears similarities to hemimegalencephaly and tuberous
scelerosis, and dysregulation of the insulin signalling mTOR/p70SK-S6 pathway (which
influences cell size and proliferation) has been shown in FCD90. The functional importance of