Comparing the change in 6-minute walk distance in nmDMD patients receiving ataluren: STRIDE Registry compared with phase 3 clinical trial
Francesco Muntoni, Már Tulinius, Filippo Buccella, Isabelle Desguerre, Janbernd Kirschner, Andrés Nascimento Osorio, Shelley Johnson, Christian Werner, Joel Jiang, James Li, Panayiota Trifillis, Eugenio Mercuri
Objectives: We investigated whether ataluren-treated nonsense mutation Duchenne muscular dystrophy (nmDMD) patients in real-world practice (STRIDE Registry; NCT02369731) experienced a similar decline in 6-minute walk distance (6MWD) vs ataluren-treated patients in a phase 3 clinical trial (Study 020; NCT01826487). The 6-minute walk test is a motor function assessment that allows progressive loss of ambulation to be recorded.
Methods: STRIDE patients (n=42) were assessed by their first 48-week change (difference between their first ‘48-week assessment’ [between 40 and 72 weeks] and first assessment); Study 020 patients (ataluren [n=45] and placebo [n=50]) were assessed by change over 48 weeks. Only patients with a 6MWD of ≥300 to ≤400 metres (m) at first assessment were assessed. For patients who lost ambulation, 6MWD was imputed as 0 m the day ambulation was lost.
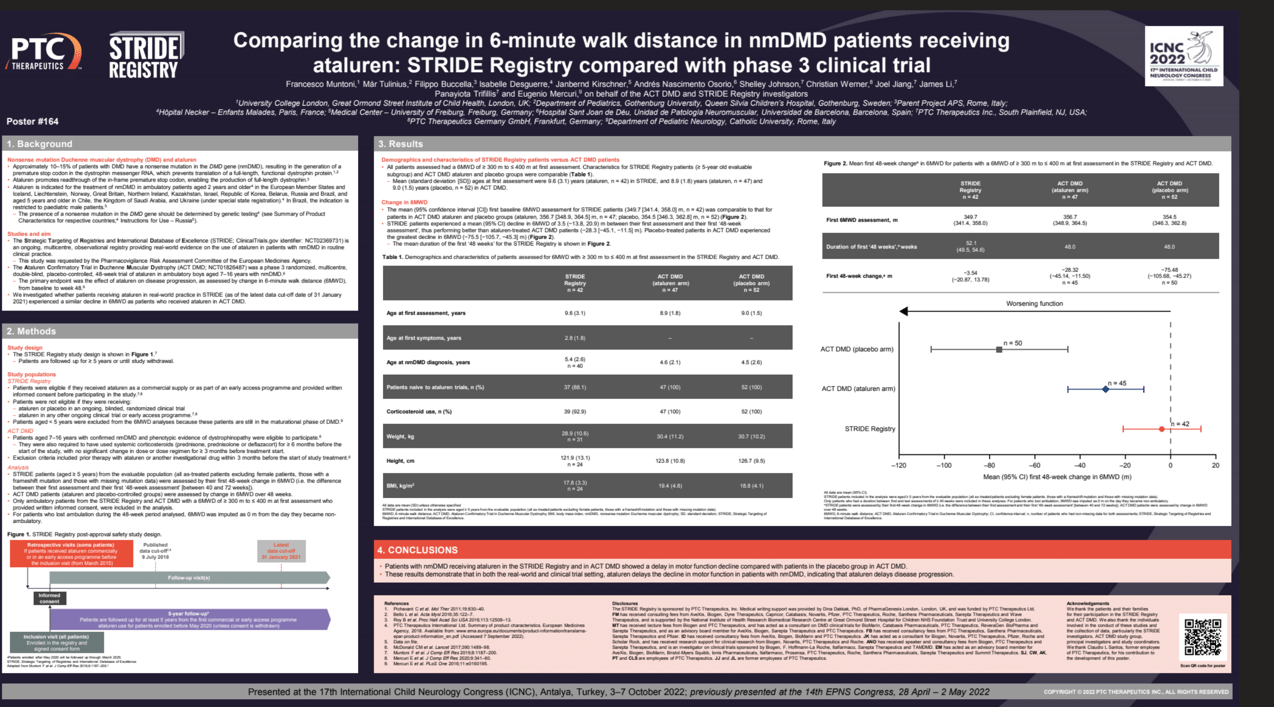
Results: Mean (95% CI) first baseline 6MWD assessment for STRIDE patients (349.7 [341.4, 358.0] m, n=42) was comparable to that for patients in Study 020 (ataluren, 356.7 [348.9, 364.5] m, n=47; placebo, 354.5 [346.3, 362.8] m, n=52). Mean (SD) age at first assessment was also comparable (STRIDE ataluren, 9.6 [3.1], n=42; 020 ataluren, 8.9 [1.8], n=47; placebo, 9.0 [1.5], n=52). STRIDE patients experienced a mean (95% CI) decline in 6MWD of −3.5 (−20.9, 13.8) m, performing better than ataluren-treated Study 020 patients (−28.3 [−45.1, −11.5] m). Placebo-allocated patients experienced a greater decline in 6MWD (−75.5 [−105.7, −45.3] m).
Conclusion: In both the real-world and clinical trial setting, ataluren delays motor function decline in nmDMD patients vs placebo, thus delaying disease progression.
Keywords: 6-minute walk distance; ataluren; Duchenne muscular dystrophy; STRIDE.
Francesco Muntoni
University College London, Great Ormond Street Institute of Child Health
United Kingdom
Már Tulinius
Gothenburg University, Queen Silvia Children’s Hospital
Sweden
Filippo Buccella
Parent Project APS
Italy
Isabelle Desguerre
Hôpital Necker – Enfants Malades
France
Janbernd Kirschner
Medical Center – University of Freiburg
Germany
Andrés Nascimento Osorio
Hospital Sant Joan de Déu, Unidad de Patología Neuromuscular, Universidad de Barcelona
Spain
Shelley Johnson
PTC Therapeutics Inc.
United States
Christian Werner
PTC Therapeutics Germany GmbH
Germany
Joel Jiang
PTC Therapeutics Inc.
United States
James Li
PTC Therapeutics Inc.
United States
Panayiota Trifillis
PTC Therapeutics Inc.
United States
Eugenio Mercuri
Catholic University
Italy
Objectives: We investigated whether ataluren-treated nonsense mutation Duchenne muscular dystrophy (nmDMD) patients in real-world practice (STRIDE Registry; NCT02369731) experienced a similar decline in 6-minute walk distance (6MWD) vs ataluren-treated patients in a phase 3 clinical trial (Study 020; NCT01826487). The 6-minute walk test is a motor function assessment that allows progressive loss of ambulation to be recorded.
Methods: STRIDE patients (n=42) were assessed by their first 48-week change (difference between their first ‘48-week assessment’ [between 40 and 72 weeks] and first assessment); Study 020 patients (ataluren [n=45] and placebo [n=50]) were assessed by change over 48 weeks. Only patients with a 6MWD of ≥300 to ≤400 metres (m) at first assessment were assessed. For patients who lost ambulation, 6MWD was imputed as 0 m the day ambulation was lost.
Results: Mean (95% CI) first baseline 6MWD assessment for STRIDE patients (349.7 [341.4, 358.0] m, n=42) was comparable to that for patients in Study 020 (ataluren, 356.7 [348.9, 364.5] m, n=47; placebo, 354.5 [346.3, 362.8] m, n=52). Mean (SD) age at first assessment was also comparable (STRIDE ataluren, 9.6 [3.1], n=42; 020 ataluren, 8.9 [1.8], n=47; placebo, 9.0 [1.5], n=52). STRIDE patients experienced a mean (95% CI) decline in 6MWD of −3.5 (−20.9, 13.8) m, performing better than ataluren-treated Study 020 patients (−28.3 [−45.1, −11.5] m). Placebo-allocated patients experienced a greater decline in 6MWD (−75.5 [−105.7, −45.3] m).
Conclusion: In both the real-world and clinical trial setting, ataluren delays motor function decline in nmDMD patients vs placebo, thus delaying disease progression.
Keywords: 6-minute walk distance; ataluren; Duchenne muscular dystrophy; STRIDE.
Francesco Muntoni
University College London, Great Ormond Street Institute of Child Health
United Kingdom
Már Tulinius
Gothenburg University, Queen Silvia Children’s Hospital
Sweden
Filippo Buccella
Parent Project APS
Italy
Isabelle Desguerre
Hôpital Necker – Enfants Malades
France
Janbernd Kirschner
Medical Center – University of Freiburg
Germany
Andrés Nascimento Osorio
Hospital Sant Joan de Déu, Unidad de Patología Neuromuscular, Universidad de Barcelona
Spain
Shelley Johnson
PTC Therapeutics Inc.
United States
Christian Werner
PTC Therapeutics Germany GmbH
Germany
Joel Jiang
PTC Therapeutics Inc.
United States
James Li
PTC Therapeutics Inc.
United States
Panayiota Trifillis
PTC Therapeutics Inc.
United States
Eugenio Mercuri
Catholic University
Italy
Panayiota Trifillis,
Francesco Muntoni, Már Tulinius, Filippo Buccella, Isabelle Desguerre, Janbernd Kirschner, Andrés Nascimento Osorio, Shelley Johnson, Christian Werner, Joel Jiang, James Li, Eugenio Mercuri
Francesco Muntoni, Már Tulinius, Filippo Buccella, Isabelle Desguerre, Janbernd Kirschner, Andrés Nascimento Osorio, Shelley Johnson, Christian Werner, Joel Jiang, James Li, Eugenio Mercuri