S-adenosylhomocysteine hydrolase deficiency with associated masseter hypertrophy, bradykinesia, and cerebellar atrophy and alterations of creatine and choline homeostasis. Expansion of cerebrohepatomuscular phenotype
Judy Pipo-Deveza, Rebekah Jobling, Susan Blaser, Amanda Carnevale, Wayne Langburt, Grace Yoon, Ingrid Tein
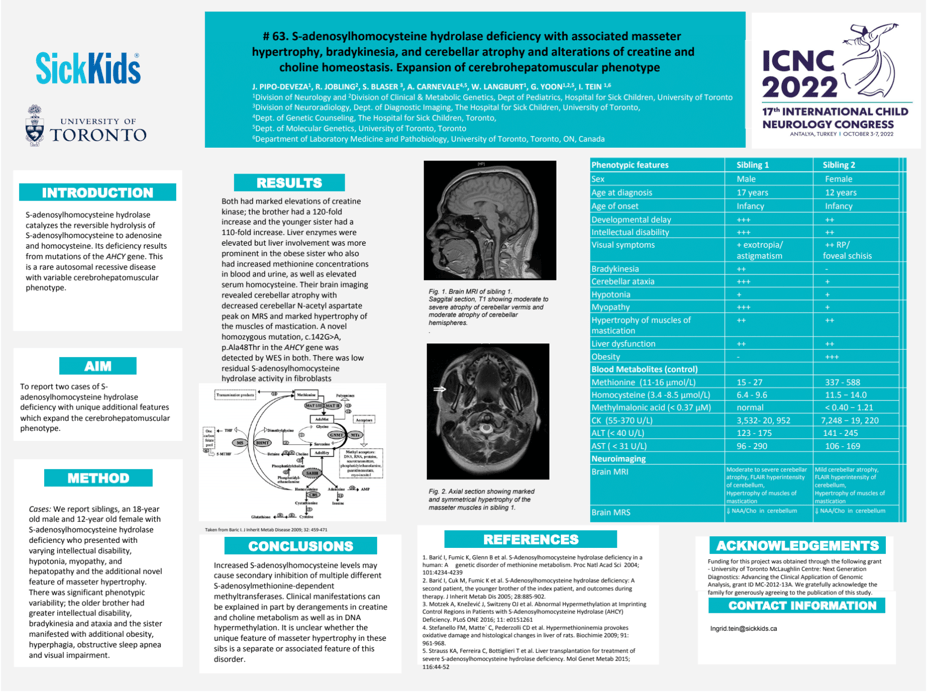
Background: S-adenosylhomocysteine hydrolase catalyzes reversible hydrolysis of S-adenosylhomocysteine to adenosine and homocysteine. Its deficiency results from mutations of the AHCY gene. This is a rare autosomal recessive disease with variable cerebrohepatomuscular phenotype. Cases: We report siblings, an 18-year old male and 12-year old female with S-adenosylhomocysteine hydrolase deficiency who presented with varying intellectual disability, hypotonia, myopathy, and hepatopathy and the additional novel feature of masseter hypertrophy. There was significant phenotypic variability; the older brother had greater intellectual disability, bradykinesia and ataxia and the sister manifested with additional obesity, hyperphagia, obstructive sleep apnea and visual impairment. Results: Both had marked elevations of creatine kinase; the brother had a 120-fold increase and the younger sister had a 110-fold increase. Liver enzymes were elevated but liver involvement was more prominent in the obese sister who also had increased methionine concentrations in blood and urine, as well as elevated serum homocysteine. Their brain imaging revealed cerebellar atrophy with decreased cerebellar N-acetyl aspartate peak on MRS and marked hypertrophy of the muscles of mastication. A novel homozygous mutation, c.142 G>A, p.Ala48Thr in the AHCY gene was detected by WES in both. There was low residual S-adenosylhomocysteine hydrolase activity in fibroblasts. Conclusions: Increased S-adenosylhomocysteine levels may cause secondary inhibition of multiple different S-adenosylmethionine-dependent methyltransferases. Clinical manifestations can be explained in part by derangements in creatine and choline metabolism as well as in DNA hypermethylation. It is unclear whether the unique feature of masseter hypertrophy in these sibs is a separate or associated feature of this disorder.
Keywords: S-adenosylhomocysteine hydrolase deficiency, hypermethioninemia, cerebellar atrophy, hyperCKemia, intellectual disability, hepatopathy
Judy Pipo-Deveza
The Hospital for Sick Children, University of Toronto
Canada
Rebekah Jobling
The Hospital for Sick Children, University of Toronto
Canada
Susan Blaser
The Hospital for Sick Children, University of Toronto
Canada
Amanda Carnevale
The Hospital for Sick Children, University of Toronto
Canada
Wayne Langburt
The Hospital for Sick Children, University of Toronto
Canada
Grace Yoon
The Hospital for Sick Children, University of Toronto
Canada
Ingrid Tein
The Hospital for Sick Children, University of Toronto
Canada
Background: S-adenosylhomocysteine hydrolase catalyzes reversible hydrolysis of S-adenosylhomocysteine to adenosine and homocysteine. Its deficiency results from mutations of the AHCY gene. This is a rare autosomal recessive disease with variable cerebrohepatomuscular phenotype. Cases: We report siblings, an 18-year old male and 12-year old female with S-adenosylhomocysteine hydrolase deficiency who presented with varying intellectual disability, hypotonia, myopathy, and hepatopathy and the additional novel feature of masseter hypertrophy. There was significant phenotypic variability; the older brother had greater intellectual disability, bradykinesia and ataxia and the sister manifested with additional obesity, hyperphagia, obstructive sleep apnea and visual impairment. Results: Both had marked elevations of creatine kinase; the brother had a 120-fold increase and the younger sister had a 110-fold increase. Liver enzymes were elevated but liver involvement was more prominent in the obese sister who also had increased methionine concentrations in blood and urine, as well as elevated serum homocysteine. Their brain imaging revealed cerebellar atrophy with decreased cerebellar N-acetyl aspartate peak on MRS and marked hypertrophy of the muscles of mastication. A novel homozygous mutation, c.142 G>A, p.Ala48Thr in the AHCY gene was detected by WES in both. There was low residual S-adenosylhomocysteine hydrolase activity in fibroblasts. Conclusions: Increased S-adenosylhomocysteine levels may cause secondary inhibition of multiple different S-adenosylmethionine-dependent methyltransferases. Clinical manifestations can be explained in part by derangements in creatine and choline metabolism as well as in DNA hypermethylation. It is unclear whether the unique feature of masseter hypertrophy in these sibs is a separate or associated feature of this disorder.
Keywords: S-adenosylhomocysteine hydrolase deficiency, hypermethioninemia, cerebellar atrophy, hyperCKemia, intellectual disability, hepatopathy
Judy Pipo-Deveza
The Hospital for Sick Children, University of Toronto
Canada
Rebekah Jobling
The Hospital for Sick Children, University of Toronto
Canada
Susan Blaser
The Hospital for Sick Children, University of Toronto
Canada
Amanda Carnevale
The Hospital for Sick Children, University of Toronto
Canada
Wayne Langburt
The Hospital for Sick Children, University of Toronto
Canada
Grace Yoon
The Hospital for Sick Children, University of Toronto
Canada
Ingrid Tein
The Hospital for Sick Children, University of Toronto
Canada
Judy Pipo-Deveza
The Hospital for Sick Children, University of Toronto Canada
The Hospital for Sick Children, University of Toronto Canada