Acute transverse myelitis related to BNT162b2 vaccine in a teenage girl
Cengiz Dilber, Sedef Terzioğlu Öztürk, Hande Dilber, Şeyma Demiray, Nursel Yurttutan, Sadık Yurttutan, Şükrü Güngör
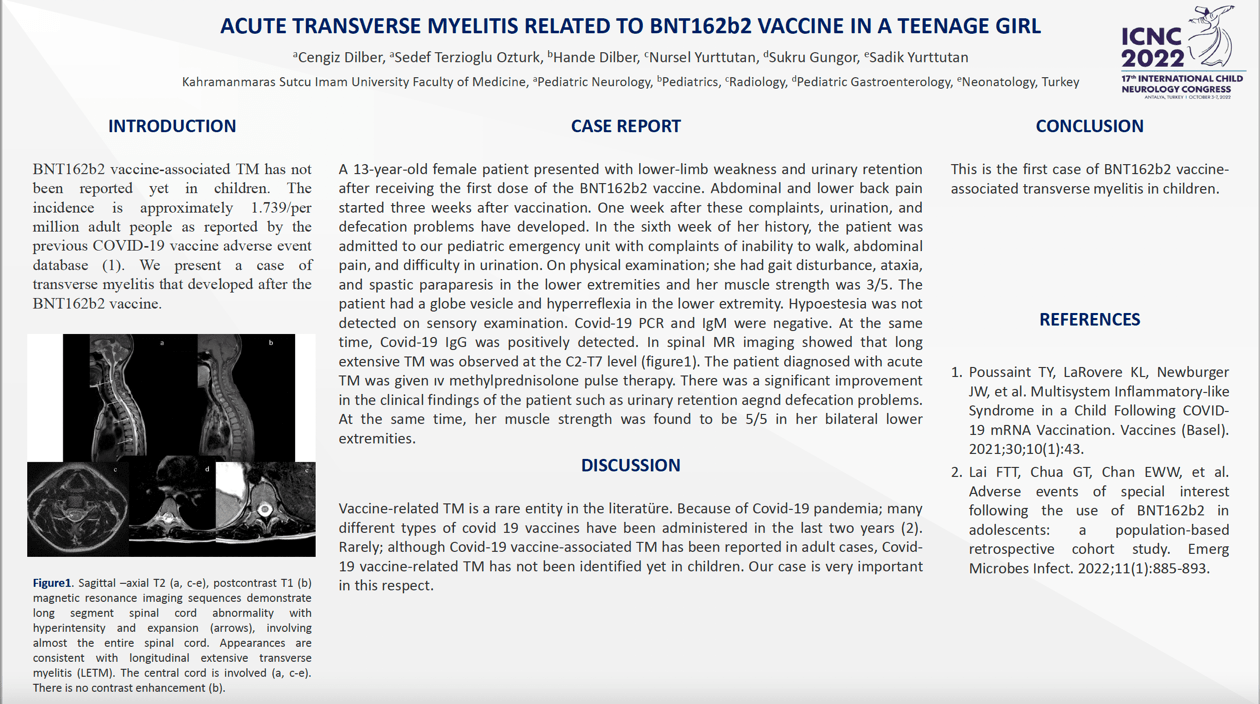
INTRODUCTION BNT162b2 vaccine-associated TM has not been reported yet in children. The incidence is approximately 1.739/per million adult people as reported by the previous COVID-19 vaccine adverse event database. We present a case of transverse myelitis that developed after the BNT162b2 vaccine. CASE REPORT A 13-year-old female patient presented with lower-limb weakness and urinary retention after receiving the first dose of the BNT162b2 vaccine. On physical examination; she had gait disturbance, ataxia, and spastic paraparesis in the lower extremities and her muscle strength was 3/5. The patient had a globe vesicle and hyperreflexia in the lower extremity. Covid-19 PCR and IgM were negative. At the same time, Covid-19 IgG was positively detected. In spinal MR imaging showed that long extensive TM was observed at the C2-T7 level. The patient diagnosed with acute TM was given ıv methylprednisolone pulse therapy. There was a significant improvement in the clinical findings of the patient such as muscle weakness, urinary retention and defecation problems. DISCUSSION: Because of Covid-19 pandemia; many different types of covid 19 vaccines have been administered in the last two years. Rarely; although Covid-19 vaccine-associated TM has been reported in adult cases, Covid-19 vaccine-related TM has not been identified yet in children. Our case is very important in this respect
Keywords:This email address is being protected from spambots. You need JavaScript enabled to view it.
Cengiz Dilber
KSU Medical Faculty
Turkey
Sedef Terzioğlu Öztürk
KSU Medical Faculty
Turkey
Hande Dilber
KSU Medical Faculty
Turkey
Şeyma Demiray
KSU Medical Faculty
Turkey
Nursel Yurttutan
KSU Medical Faculty
Turkey
Sadık Yurttutan
KSU Medical Faculty
Turkey
Şükrü Güngör
KSU Medical Faculty
Turkey
INTRODUCTION BNT162b2 vaccine-associated TM has not been reported yet in children. The incidence is approximately 1.739/per million adult people as reported by the previous COVID-19 vaccine adverse event database. We present a case of transverse myelitis that developed after the BNT162b2 vaccine. CASE REPORT A 13-year-old female patient presented with lower-limb weakness and urinary retention after receiving the first dose of the BNT162b2 vaccine. On physical examination; she had gait disturbance, ataxia, and spastic paraparesis in the lower extremities and her muscle strength was 3/5. The patient had a globe vesicle and hyperreflexia in the lower extremity. Covid-19 PCR and IgM were negative. At the same time, Covid-19 IgG was positively detected. In spinal MR imaging showed that long extensive TM was observed at the C2-T7 level. The patient diagnosed with acute TM was given ıv methylprednisolone pulse therapy. There was a significant improvement in the clinical findings of the patient such as muscle weakness, urinary retention and defecation problems. DISCUSSION: Because of Covid-19 pandemia; many different types of covid 19 vaccines have been administered in the last two years. Rarely; although Covid-19 vaccine-associated TM has been reported in adult cases, Covid-19 vaccine-related TM has not been identified yet in children. Our case is very important in this respect
Keywords:
Cengiz Dilber
KSU Medical Faculty
Turkey
Sedef Terzioğlu Öztürk
KSU Medical Faculty
Turkey
Hande Dilber
KSU Medical Faculty
Turkey
Şeyma Demiray
KSU Medical Faculty
Turkey
Nursel Yurttutan
KSU Medical Faculty
Turkey
Sadık Yurttutan
KSU Medical Faculty
Turkey
Şükrü Güngör
KSU Medical Faculty
Turkey
Sedef Terzioğlu Öztürk
KSU Medical Faculty Turkey
KSU Medical Faculty Turkey