Acute Treatment of Migraine In Pre-Adolescents: Real-World Analysis Of Remote Electrical Neuromodulation (REN)
Objective: Migraine impacts both children and adolescents, causing significant disability. However, only a single pharmacological acute treatment is approved for ages 6-12 years, resulting in off-label prescriptions for other treatments. Remote Electrical Neuromodulation(REN) is a non-pharmacological, prescribed, wearable device, FDA-cleared for acute and/or preventive treatment of migraine with or without aura in patients 12 years or older. While prior studies demonstrated REN's safety and efficacy in adolescents (12-17 years), this study aims to assess its real-world safety and efficacy in pre-adolescents aged 9-11.
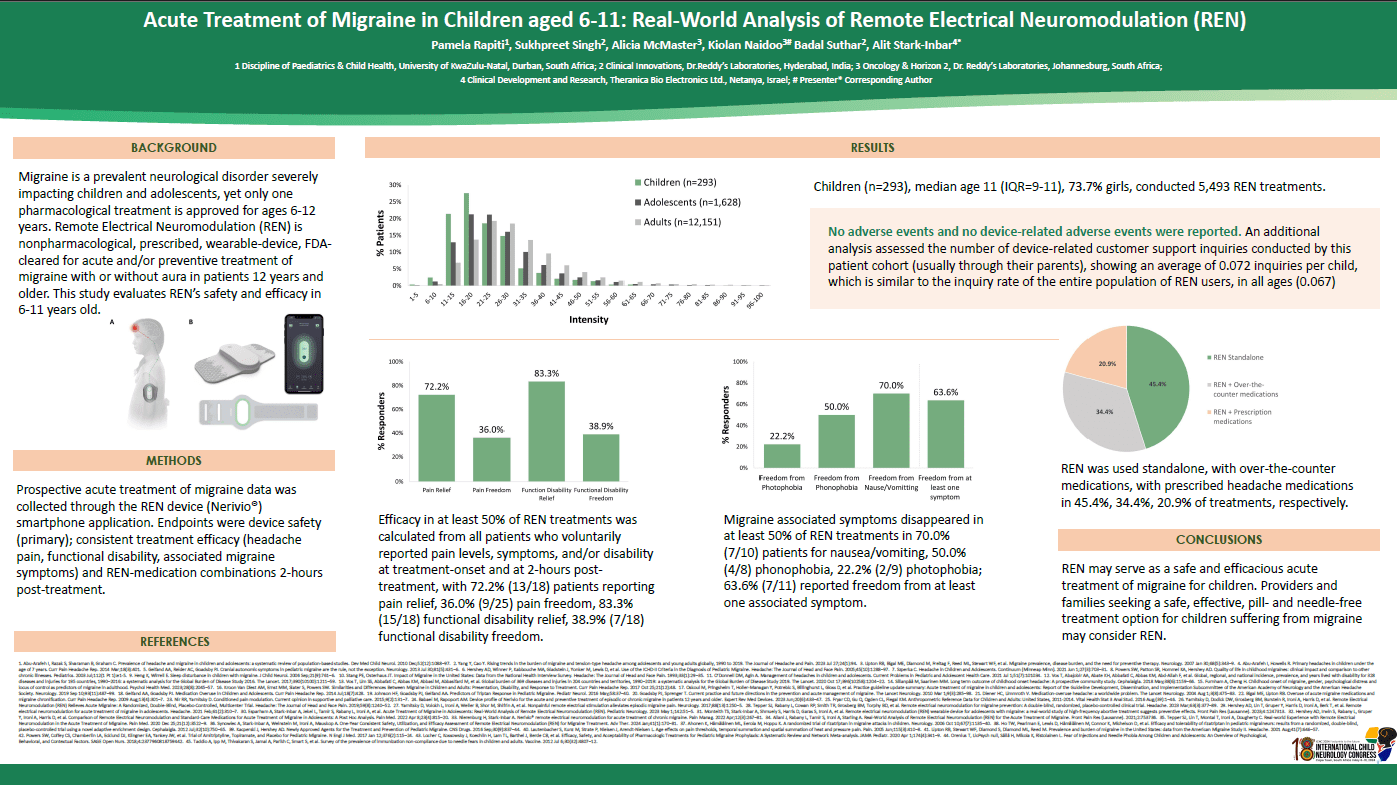
Methods: Prospective data on pre-adolescent users who used REN at least once between May 2020 and June 2023 was collected through the REN device (Nerivio®) smartphone application. Device safety was measured using an adverse event tracking system. Efficacy was measured in patients who voluntarily reported prospective migraine symptoms at treatment onset and 2 hours later.
Results: The study involved 52 pre-adolescents, mean age 10.9±0.73 years. Median treatments: 9.0; average intensity: 25.4%. No adverse events were reported. After the first REN treatment, 60% achieved 2-hour pain relief, and 40% attained pain freedom. Consistent pain relief and freedom in at least 50% of treatments: 78% and 55%, respectively. Moreover, REN avoided prescription drugs in 82% of treatments.
Conclusion: REN's efficacy and safety in pre-adolescents align with findings in adolescents. So providers looking for a safe, effective option for migraine in this age group may consider REN as a novel treatment.
Pamela Rapiti
Clinical Paediatric Neurologist
Durban, South Africa
Sukhpreet Singh
Clinical Innovation Lead
PortMgt,Global Strt Mktg&EarlyStage Part
Dr. Reddy's Laboratories Ltd.
Hyderabad, India
Alicia McMaster
Head of Medical: Dr Reddy’s South Africa
Medical Affairs
Dr. Reddy's Laboratories Ltd.
Johannesburg, South Africa
Badal Suthar
Medical Advisor - Clinical Innovations
PortMgt,Global Strt Mktg&EarlyStage Part
Dr. Reddy's Laboratories Ltd.
Hyderabad, India
Alit Stark-Inbar
Vice President of Medical Information
Clinical Development and Research (Clinical R&D)
Theranica Bio Electronics Ltd.
Netanya, Israel