Safety And Efficacy Of Adjunctive Fenfluramine In An Open-Label Extension Study Of Children (Under 6 Years Old) With Dra
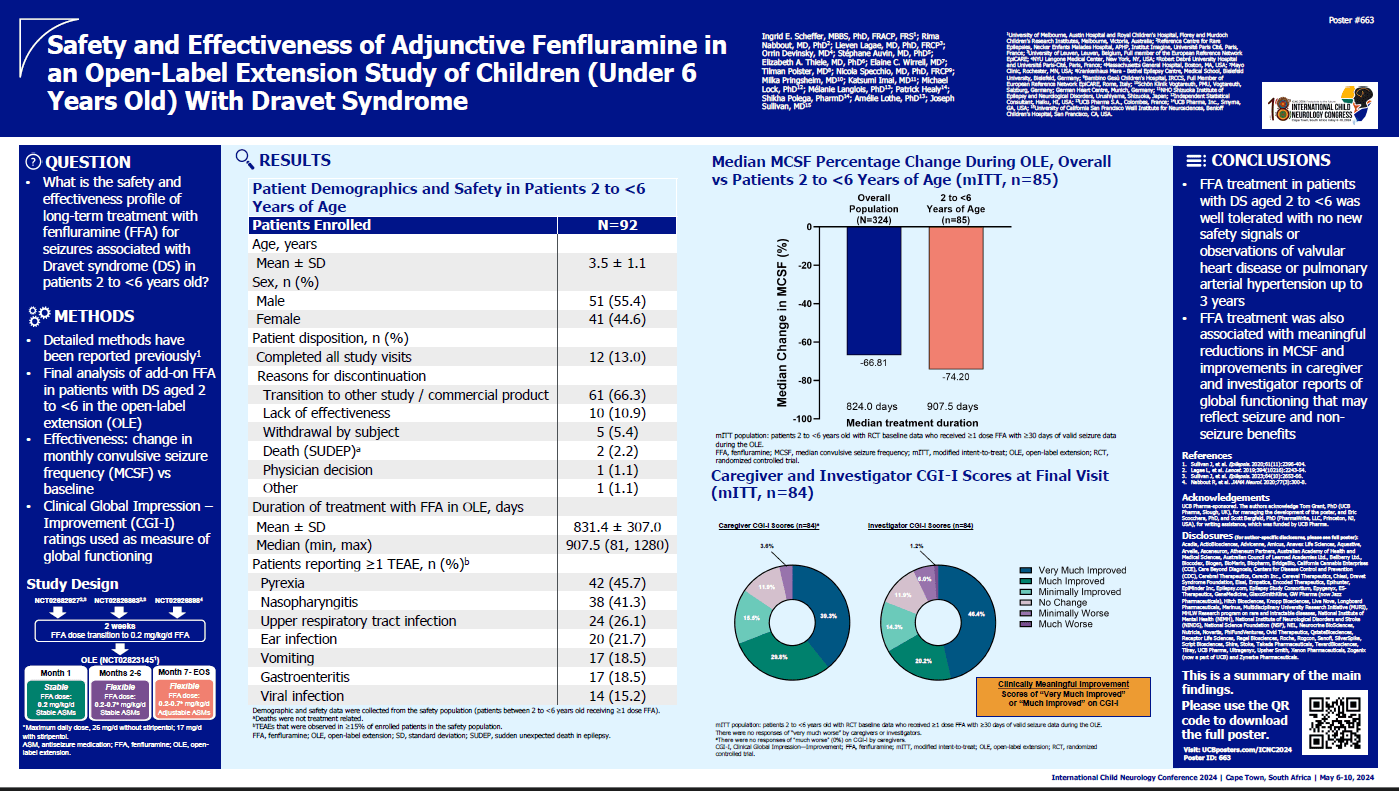
Introduction: Phase 3 studies and interim open-label extension (OLE) analyses have shown profound, durable reduction in median monthly convulsive seizure frequency (MCSF) in patients with Dravet syndrome (DS) treated with fenfluramine (FFA). This final OLE analysis describes long-term safety and efficacy of FFA in patients under 6 years of age with DS.
Methods: Safety was reported for patients who received >=1 dose FFA. The key efficacy endpoint in the modified intent-to-treat (mITT) patients was change in median MCSF from core baseline to overall OLE. Caregivers and investigators rated patients on the Clinical Global Impression-Improvement (CGI-I) scale. Descriptive statistics and Wilcoxon test for % MCSF change from baseline were used.
Results: As of 27 Jan 2023, 375 patients had enrolled; 92 were 2-6 years old and received >=1 dose FFA (Table 1, demographics and exposure). In the mITT population (n=85), median MCSF change from baseline during the overall OLE was -74.2%, P<0.001. Clinically meaningful improvement (much improved or very much improved) was reported by 69.0% and 66.7% of caregivers and investigators, respectively. There were no new or unexpected treatment-emergent adverse events (Table 2) and no cases of valvular heart disease or pulmonary hypertension.
Conclusion: Patients with DS aged <6 years treated with FFA (median, 2.5 y) have sustained reduction in seizures with clinically meaningful improvement in severity of condition.
Ingrid E Scheffer
University of Melbourne, Austin Hospital and Royal Children's Hospital, Florey and Murdoch Children’s Research Institutes
Australia
Rima Nabbout
Reference Centre for Rare Epilepsies, Necker Enfants Malades Hospital, APHP, Institut Imagine, Université Paris Cité
France
Lieven Lagae
University of Leuven, Full member of the European Reference Network EpiCARE
Belgium
Orrin Devinsky
NYU Langone Medical Center
United States
Stéphane Auvin
Robert Debré University Hospital and Université Paris-Cité
France
Elizabeth A Thiele
Massachusetts General Hospital
United States
Elaine C Wirrell
Mayo Clinic
United States
Tilman Polster
Krankenhaus Mara - Bethel Epilepsy Centre, Medical School, Bielefeld University
Germany
Nicola Specchio
Bambino Gesù Children's Hospital, IRCCS, Full Member of European Reference Network EpiCARE
Italy
Milka Pringsheim
Schön Klinik Vogtareuth, PMU
Germany
Katsumi Imai
NHO Shizuoka Institute of Epilepsy and Neurological Disorders
Japan
Michael Lock
Independent Statistical Consultant
United States
Mélanie Langlois
UCB Pharma S.A.
France
Patrick Healy
UCB Pharma, Inc.
United States
Shikha Polega
UCB Pharma, Inc.
United States
Amélie Lothe
UCB Pharma S.A.
France
Joseph Sullivan
University of California San Francisco Weill Institute for Neurosciences, Benioff Children’s Hospital
United States
Nicola Specchio
Bambino Gesù Children's Hospital, IRCCS, Full Member of European Reference Network EpiCARE
Italy