Utilization Of Intravenous Immunoglobulin In The Neurology Department Of A Middle Eastern Tertiary Pediatric Hospital
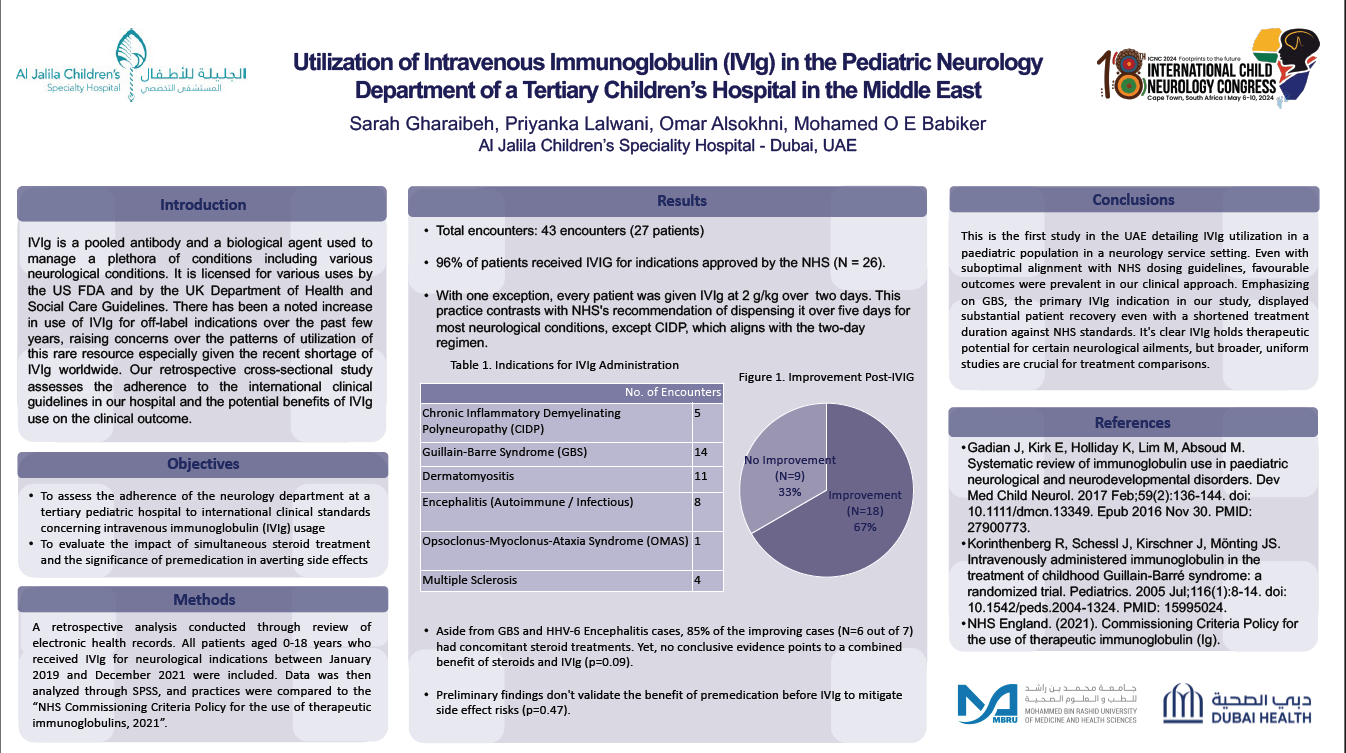
Objectives: This study aims to assess the adherence of the neurology department at our pediatric hospital to global clinical standards concerning intravenous immunoglobulin (IVIg) usage. The investigation also delves into the impact of simultaneous steroid treatment and the significance of premedication in averting side effects.
Methods: A retrospective study at Al Jalila Children’s Hospital examined children under 18 receiving IVIg for neurological conditions from 2019 to 2021. Data was analyzed using SPSS software and compared against the 2021 NHS guidelines for therapeutic immunoglobulin use.
Results: Over three years, the neurology department treated 27 patients with IVIg in 43 encounters. 96% received IVIg for NHS-approved conditions, with GBS being the main indication (40% of cases). The typical dosage was 2 g/kg over two days, contrasting NHS's general five-day guideline. One encounter for multiple sclerosis, an unapproved indication by NHS, yielded no improvement. Overall, 66% improved after IVIg. Notably, 81% of GBS cases showed significant recovery. Excluding GBS and HHV-6 Encephalitis, 85% of improved cases had simultaneous steroid treatments, but no evident synergistic effect with IVIg was found. Of note, preliminary data didn't confirm the efficacy of premedication to reduce IVIg side effects.
Conclusion: The first UAE study on IVIg use in a pediatric neurology department showed positive results despite not fully aligning with NHS guidelines. Focusing on GBS, patients recovered well with shorter treatments. IVIg is beneficial for some neurological conditions, but more comprehensive studies are needed for comparisons.
Sarah Gharaibeh
Al Jalila Children's Hospital
United Arab Emirates
Priyanka Lalwani
Al Jalila Children's Hospital
United Arab Emirates
Omar Al Sokhni
Al Jalila Children's Hospital
United Arab Emirates
Mohamed O E Babiker
Al Jalila Children's Hospital
United Arab Emirates