Updated demographics and safety data from patients with nonsense mutation Duchenne muscular dystrophy receiving ataluren in the STRIDE Registry
Francesco Muntoni, Filippo Buccella, Isabelle Desguerre, Janbernd Kirschner, Eugenio Mercuri, Andrés Nascimento Osorio, Már Tulinius, Shelley Johnson, Christian Werner, Joel Jiang, James Li, Panayiota Trifillis
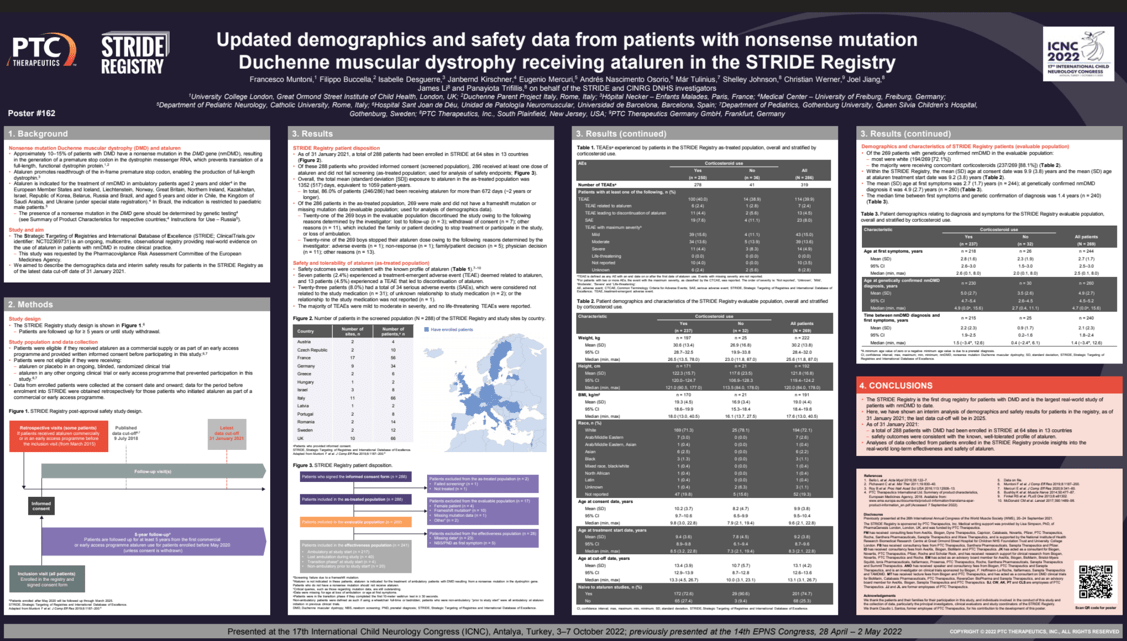
Objectives: Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder caused by a lack of functional dystrophin. Ataluren is indicated for treatment of patients with nonsense mutation (nm) DMD. Strategic Targeting of Registries and International Database of Excellence (STRIDE; NCT02369731) is an ongoing registry providing real-world data on ataluren use in patients with nmDMD. Here, we describe STRIDE population demographics and interim safety results, as of the data cut-off of January 31, 2021.
Methods: Data from enrolled patients are collected at the consent date; data for patients who initiated ataluren before enrollment are obtained retrospectively. Patients will be followed up for ≥5 years or until study withdrawal.
Results: As of January 31, 2021, 286 boys had been enrolled and received at least one ataluren dose. Total mean (SD) ataluren exposure was 1352 (517) days; equivalent to 1059 patient-years. Safety outcomes were consistent with the known safety profile of ataluren. Thirty-one of the 286 boys discontinued the study. nmDMD was genetically confirmed in 269/286 boys. Most patients were Caucasian (194/269 [72.1%]); mean (SD) age at consent was 9.9 (3.8) years (n=269). Mean (SD) age at first symptoms was 2.7 (1.7) years (n=244); at nmDMD confirmation, it was 4.9 (2.7) years (n=260). Median time between first symptoms and nmDMD confirmation was 1.4 years (n=240). Most patients used concomitant corticosteroids (237/269 [88.1%]).
Conclusion: STRIDE is the first drug registry for nmDMD patients. Interim data suggest that ataluren has a favorable safety profile in routine clinical practice, consistent with that shown in clinical trials.
Keywords: ataluren; Duchenne muscular dystrophy; STRIDE.
Francesco Muntoni
University College London, Great Ormond Street Institute of Child Health
United Kingdom
Filippo Buccella
Parent Project APS
Italy
Isabelle Desguerre
Hôpital Necker – Enfants Malades
France
Janbernd Kirschner
University of Freiburg
Germany
Eugenio Mercuri
Catholic University
Italy
Andrés Nascimento Osorio
Universidad de Barcelona
Spain
Már Tulinius
Gothenburg University, Queen Silvia Children’s Hospital
Sweden
Shelley Johnson
PTC Therapeutics Inc.
United States
Christian Werner
PTC Therapeutics Germany GmbH
Germany
Joel Jiang
PTC Therapeutics Inc.
United States
James Li
PTC Therapeutics Inc.
United States
Panayiota Trifillis
PTC Therapeutics Inc.
United States
Objectives: Duchenne muscular dystrophy (DMD) is a severe neuromuscular disorder caused by a lack of functional dystrophin. Ataluren is indicated for treatment of patients with nonsense mutation (nm) DMD. Strategic Targeting of Registries and International Database of Excellence (STRIDE; NCT02369731) is an ongoing registry providing real-world data on ataluren use in patients with nmDMD. Here, we describe STRIDE population demographics and interim safety results, as of the data cut-off of January 31, 2021.
Methods: Data from enrolled patients are collected at the consent date; data for patients who initiated ataluren before enrollment are obtained retrospectively. Patients will be followed up for ≥5 years or until study withdrawal.
Results: As of January 31, 2021, 286 boys had been enrolled and received at least one ataluren dose. Total mean (SD) ataluren exposure was 1352 (517) days; equivalent to 1059 patient-years. Safety outcomes were consistent with the known safety profile of ataluren. Thirty-one of the 286 boys discontinued the study. nmDMD was genetically confirmed in 269/286 boys. Most patients were Caucasian (194/269 [72.1%]); mean (SD) age at consent was 9.9 (3.8) years (n=269). Mean (SD) age at first symptoms was 2.7 (1.7) years (n=244); at nmDMD confirmation, it was 4.9 (2.7) years (n=260). Median time between first symptoms and nmDMD confirmation was 1.4 years (n=240). Most patients used concomitant corticosteroids (237/269 [88.1%]).
Conclusion: STRIDE is the first drug registry for nmDMD patients. Interim data suggest that ataluren has a favorable safety profile in routine clinical practice, consistent with that shown in clinical trials.
Keywords: ataluren; Duchenne muscular dystrophy; STRIDE.
Francesco Muntoni
University College London, Great Ormond Street Institute of Child Health
United Kingdom
Filippo Buccella
Parent Project APS
Italy
Isabelle Desguerre
Hôpital Necker – Enfants Malades
France
Janbernd Kirschner
University of Freiburg
Germany
Eugenio Mercuri
Catholic University
Italy
Andrés Nascimento Osorio
Universidad de Barcelona
Spain
Már Tulinius
Gothenburg University, Queen Silvia Children’s Hospital
Sweden
Shelley Johnson
PTC Therapeutics Inc.
United States
Christian Werner
PTC Therapeutics Germany GmbH
Germany
Joel Jiang
PTC Therapeutics Inc.
United States
James Li
PTC Therapeutics Inc.
United States
Panayiota Trifillis
PTC Therapeutics Inc.
United States