Eladocagene exuparvovec gene therapy improves motor development in patients with aromatic L-amino acid decarboxylase deficiency
Paul Wuh-Liang Hwu, Yin-Hsiu Chien, Ni-Chung Lee, Sheng-Hong Tseng, Antonia Wang, Panayiota Trifillis, Tuna Koca, Chun-Hwei Tai
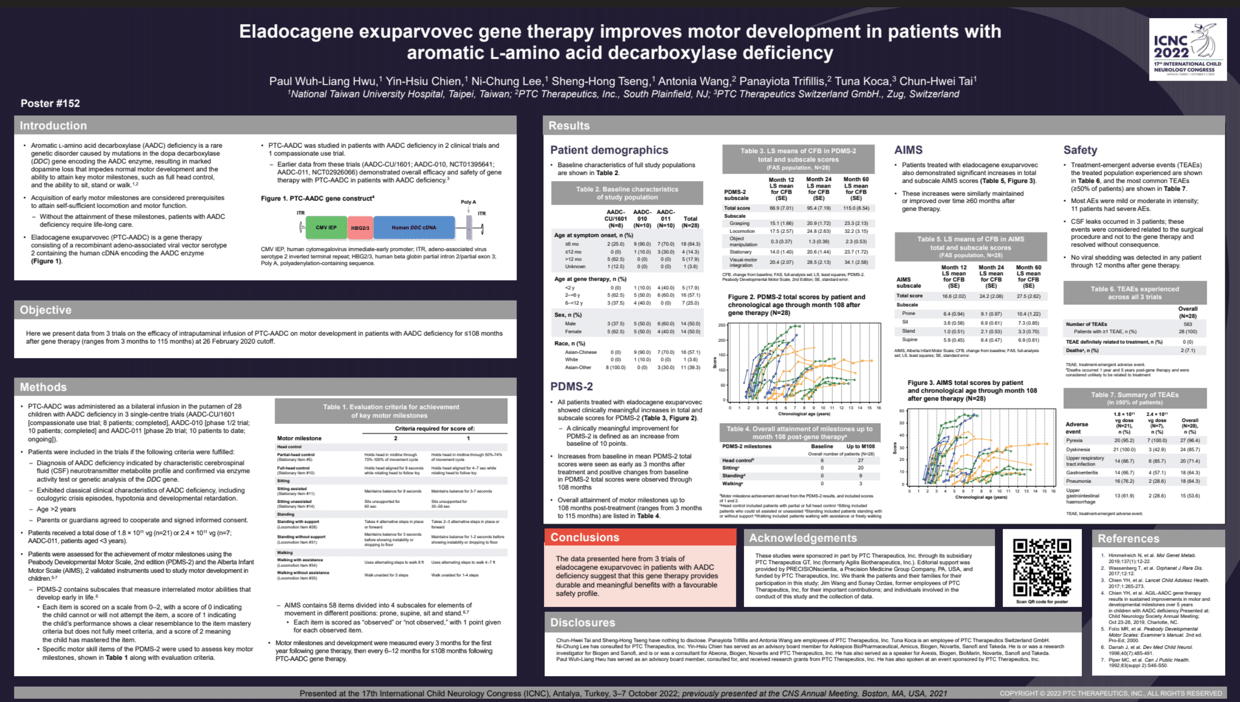
Objectives: Aromatic L-amino acid decarboxylase (AADC) deficiency is caused by mutations in the dopa decarboxylase gene leading to reduced AADC enzyme activity. This condition is characterized by motor impairments and inability to attain key developmental milestones. Eladocagene exuparvovec is a recombinant adeno-associated viral vector serotype 2 carrying the coding sequence for human AADC.
Methods: Eladocagene exuparvovec was administered via bilateral infusions to the putamen of 28 children with AADC deficiency in 3 clinical trials (AADC-CU/1601 [8 patients, completed], AADC-010 [10 patients, completed], and AADC-011 [10 patients at 26 February 2020 cutoff, ongoing]). Patients received a total of 1.8 × 10^11 vg (n=21) or 2.4 × 10^11 vg (n=7; AADC-011)]. Patients were assessed for the achievement of motor milestones using the Peabody Developmental Motor Scale, 2nd edition (PDMS-2) and Alberta Infant Motor Scale (AIMS). PDMS-2 and AIMS are validated instruments used to study motor development in children. PDMS-2 contains subscales for interrelated motor abilities, and AIMS contains subscales for elements of movement in different positions.
Results: All patients treated with eladocagene exuparvovec showed clinically meaningful increases in total and subscale scores for PDMS-2 and increases in AIMS scores, which were maintained or improved over time up to 60 months (Tables 1 and 2). Clinically meaningful increases from baseline in PDMS-2 total scores were seen as early as 3 months after treatment and extended to at least 60 months.
Conclusions: The data indicate that eladocagene exuparvovec can provide a durable positive impact on motor development in patients with AADC deficiency.
Keywords: Rare Diseases, AADC Deficiency, Gene Therapy, Movement Disorders
Paul Wuh-Liang Hwu
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Yin-Hsiu Chien
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Ni-Chung Lee
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Sheng-Hong Tseng
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Antonia Wang
PTC Therapeutics, Inc.
United States
Panayiota Trifillis
PTC Therapeutics, Inc.
United States
Tuna Koca
PTC Therapeutics, Inc.
Switzerland
Chun-Hwei Tai
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Objectives: Aromatic L-amino acid decarboxylase (AADC) deficiency is caused by mutations in the dopa decarboxylase gene leading to reduced AADC enzyme activity. This condition is characterized by motor impairments and inability to attain key developmental milestones. Eladocagene exuparvovec is a recombinant adeno-associated viral vector serotype 2 carrying the coding sequence for human AADC.
Methods: Eladocagene exuparvovec was administered via bilateral infusions to the putamen of 28 children with AADC deficiency in 3 clinical trials (AADC-CU/1601 [8 patients, completed], AADC-010 [10 patients, completed], and AADC-011 [10 patients at 26 February 2020 cutoff, ongoing]). Patients received a total of 1.8 × 10^11 vg (n=21) or 2.4 × 10^11 vg (n=7; AADC-011)]. Patients were assessed for the achievement of motor milestones using the Peabody Developmental Motor Scale, 2nd edition (PDMS-2) and Alberta Infant Motor Scale (AIMS). PDMS-2 and AIMS are validated instruments used to study motor development in children. PDMS-2 contains subscales for interrelated motor abilities, and AIMS contains subscales for elements of movement in different positions.
Results: All patients treated with eladocagene exuparvovec showed clinically meaningful increases in total and subscale scores for PDMS-2 and increases in AIMS scores, which were maintained or improved over time up to 60 months (Tables 1 and 2). Clinically meaningful increases from baseline in PDMS-2 total scores were seen as early as 3 months after treatment and extended to at least 60 months.
Conclusions: The data indicate that eladocagene exuparvovec can provide a durable positive impact on motor development in patients with AADC deficiency.
Keywords: Rare Diseases, AADC Deficiency, Gene Therapy, Movement Disorders
Paul Wuh-Liang Hwu
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Yin-Hsiu Chien
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Ni-Chung Lee
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Sheng-Hong Tseng
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Antonia Wang
PTC Therapeutics, Inc.
United States
Panayiota Trifillis
PTC Therapeutics, Inc.
United States
Tuna Koca
PTC Therapeutics, Inc.
Switzerland
Chun-Hwei Tai
National Taiwan University Hospital and National Taiwan University College of Medicine
Taiwan
Tuna Koca
PTC Therapeutics, Inc. Switzerland
PTC Therapeutics, Inc. Switzerland