Efficacy and Safety of Cannabidiol Dose Adjustment in Patients with Lennox-Gastaut Syndrome in a Phase 3 Trial and Open-label Extension
Timothy B Saurer, Elaine C Wirrell, Ashley Schreiber, Robert T Wechsler
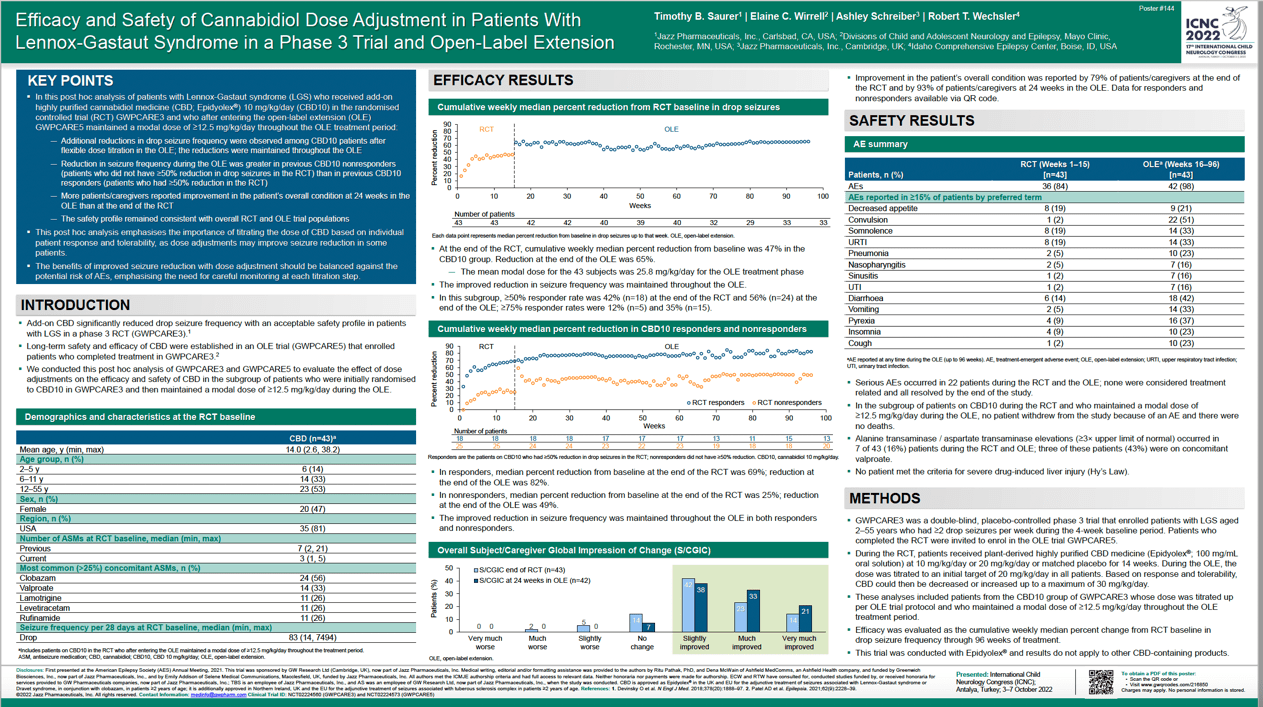
Objectives: A Phase 3 randomised controlled trial (RCT; NCT02224560) and open-label extension (OLE; NCT02224573) demonstrated the long-term safety and efficacy of cannabidiol (CBD) in reducing drop seizure frequency in patients with Lennox-Gastaut syndrome (LGS). This post-hoc analysis evaluated the effect of dose adjustments on the efficacy and safety of CBD.
Methods: Patients received plant-derived highly purified CBD medicine (Epidyolex®; 100 mg/mL oral solution) at 10 mg/kg/day (CBD10), 20 mg/kg/day or matched placebo for 14 weeks (RCT). Patients who completed the RCT could enrol in the OLE, with CBD initially titrated to 20 mg/kg/day and subsequent adjustments based on response and tolerability (maximum 30 mg/kg/day). Analyses included patients receiving CBD10 with doses titrated up in the OLE and maintained at a modal dose of ≥12.5 mg/kg/day. Weekly median percent change from baseline in drop seizure frequency was assessed through 96 weeks.
Results: Analyses included 43 OLE patients; median (range) age: 12 (3–38) years. Cumulative weekly median seizure reduction: 47% (end of RCT), reduced by a further 14% (OLE) and maintained throughout the trial. Seizure frequency reduction during the OLE was greater in patients (58%) with <50% reduction during the RCT versus patients (42%) with ≥50% reduction. AE incidence: 84% (RCT); 98% (OLE). Most common AEs: somnolence, decreased appetite and upper respiratory tract infection (19% each; RCT); convulsion (51%), diarrhoea (42%) and pyrexia (37%; OLE).
Conclusion: This post-hoc analysis emphasises the importance of titrating to each patient’s therapeutic dose, which may improve seizure reduction in some patients with LGS.
Keywords: Lennox-Gastaut syndrome, Cannabidiol, Post hoc, Dos
Timothy B Saurer
Jazz Pharmaceuticals
United States
Elaine C Wirrell
Divisions of Child and Adolescent Neurology and Epilepsy
United States
Ashley Schreiber
Jazz Pharmaceuticals
United Kingdom
Robert T Wechsler
Idaho Comprehensive Epilepsy Center
United States